Acids and Bases
advertisement

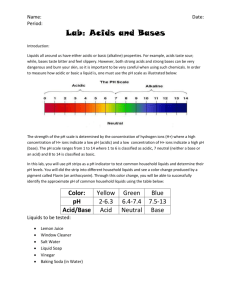
Acids and Bases Some people drink a full glass of acid at breakfast every morning. Do you? If you drink orange juice, then you’re drinking acid. When you hear acids and bases, you might think of something dangerous or something that only mad scientists use. This is not true. Acids and bases are everywhere: in the food you eat, in your house, in super markets, and even inside your body! So let’s see what acids and bases really are. Acid Most things that taste sour are acids. Acids have different strengths. The stronger the acid, the better it is at dissolving things. For example, acid in your stomach (hydrochloric acid) is so strong that it breaks down the food you eat. Also, you get tooth cavities because germs feed on bits of food that you forget to brush off and make a strong acid that melts your teeth. Base Bases are the opposites of acids (you will see what this means a little later). Like acids, bases have different strengths, and stronger bases dissolve things better than weaker ones. Strong bases are commonly used to clean water pipes and other surfaces. Neutral So acids and bases are opposites of each other. What this means is that when you mix an acid and a base that are equally strong, they cancel each other out. This is called neutralization. Neutralization makes something that is neither acidic nor basic; this is called neutral. Pure water is neutral. Experiments Egg Experiment: It’s time to see what happened to our eggs from last week! Have your mentor give you and your partner your box and observe what happened to your egg. Why do you think the shell disappeared? How would you tell if something is an acid or base? Some weak acids like orange juice and lemonade taste sour. Some weak bases, like soap, feel slippery to the touch. But strong acids and bases are dangerous, so we can’t touch or taste to tell if something is an acid or base. But don’t worry, we have something called indicators. Indicators are chemicals that change color in acids and bases. We will use an indicator made from red cabbage. When red cabbage juice (indicator) is added to an acid, the liquid turns pink. When it is added to a base, it turns blue-green. When it is added to a neutral liquid, it does not change color. So by the color of the indicator, you can tell if something is an acid or a base. Neutralization Experiment: This week during our testing for acids and bases we have acidic and basic controls. We will start with an experiment using both of our controls. Materials: Red cabbage juice Droppers Vinegar Aluminum Foil (spread on desk) Baking Soda Cups Method: 1. Place a small amount of baking soda in the cup and add a few drops of cabbage juice. Note the color. 2. Do the same in another cup with the vinegar. These are your positive controls for base (baking soda) and acid (vinegar). 3. Add a few drops of cabbage juice to water. This is your negative control for acids and bases. 4. Now you are going to neutralize your baking soda with acid. Add vinegar to your baking soda and cabbage juice cup until the color of the indicator changes. How do you know when it is neutral? How do you know when it has become acidic? Indicator Testing Materials: Red cabbage juice Detergent Vitamin C Lime Juice Honey Tums Cornstarch Soda Egg Whites Droppers Cups Aluminum Foil (spread on desk) Method: 1. Get a small portion of each unknown. Add a few drops of indicator solution and note the color change, if any. 2. Fill out the chart below with as many unknowns as you can. Unknown Indicator Color Acid, Base or Neutral? Vinegar Baking Soda Water Detergent Vitamin C Lime Juice Honey Tums Cornstarch Soda Egg White Questions 1. When milk rots, is it getting more acidic or basic? 2. Why is acid rain bad? 3. What would happen if you put sprinkle baking soda onto sweet and sour pork? Why do you put baking soda into cake dough? 4. You get a heart burn when your stomach makes too much acid. What kind of drugs can you take to cure this (acid or base)?