07a. Polar & Non-Polar expt_21oct13a
advertisement

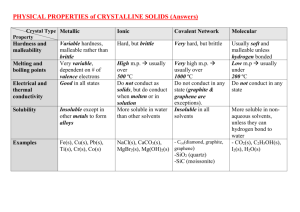
Chemistry 32A Cover sheet for Polar & Non-Polar Materials Student name (please print) ____________________________________________ Lab section, day and time ____________________________________________ Date report submitted ____________________________________________ Provide your assessment of the experiment, some suggestions: What did you learn from the experiment? Please be specific Were instructions clear and/or easy to follow, sufficient time for completion? Highlights and/or lowlights? Suggestions for improvement? Use the space below, or attach comments on a separate sheet POLAR AND NON-POLAR MATERIALS Solubility of one material (solute) in another (solvent) depends on compatibility of molecular structure between the two, often summarized by “like dissolves like”. Materials with no electrical polarity are referred to as “nonpolar”, examples of which include atmospheric gases, oils, and many organic solvents. Other materials have molecular polarity, meaning a charge separation resulting in either charged particles as ions or asymmetric molecules having a positive and negative end, similar to the north and south poles of a bar magnet. The separation of charge is due to an element’s electro negativity, with Fluorine being the most negative, meaning it has the strongest ability to attract electrons. This unequal electron attraction results in ions or molecules with positive and negative ends. These are “Polar” and have the property of mixing with other polar materials, leading to the generalization that “like dissolves like”. Water is the most common polar material and dissolves most other polar materials, such as salt and sugar. Materials which do not have a separation of charge, or have charges which are evenly balanced are considered “nonpolar”. Polarity is symbolized by the small Greek letter delta (δ) with a plus or minus sign added to indicate charge. The charge direction can also be indicated with an arrow, which head faces the negative end of the molecule and the other end has a cross indicating the positive end. In the process of dissolving in water, polar materials often generate ions, which are charged molecular species surrounded by water molecules, illustrated below. This ionic solution can conduct an electric current similar to electrons in a wire. \ Some materials are “in between”, being polar (dissolve in water) but do not conduct an electric current. These are called Polar Covalent, an example of which is Sucrose ... ordinary table sugar. Solubility also depends on temperature. Our everyday experience is that things dissolve more quickly or completely in hot rather than cold water, so we wash dishes and clothes using hot water to accelerate the cleaning action. For gases, the reverse is true. Oxygen and Carbon Dioxide are more soluble in cold water. PROCEDURES Part A: Solubility in Polar (water) and Non-Polar (hexane) solvents You will evaluate the solubility of 12 solutes in two solvents, for a total of 24 samples. In the case of water you will also do a conductivity test using an LED indicator attached to a battery. If the light glows, it indicates conductivity. 1. Label 12 test tubes with the solute to be tested; then fill about 1/3 with distilled or deionized water after making sure they are clean and rinsed with deionized water. You must NOT use tap water which has enough dissolved salts to be electrically conductive without added solutes. Do the same with 12 more test tubes and fill about 1/3 with hexane. 2. Add a small amount of each solute to the respective labeled test tubes containing water or hexane. An amount the size of a few grains of rice is sufficient. Swirl or tap the test tubes and observe if the solute is dissolving. Solutes should dissolve in one solvent but not the other. In some cases a strong color will confirm dissolving. For vitamin E, it will be necessary to cut the end off a capsule and squeeze a drop of oil into the test tubes. 3. Decide which materials are soluble in which solvents and enter the data on your lab manual data sheet. 4. For solutes in water, use the conductivity probe to see if the solution is electrically conductive or not. Relative conductivity is shown by brightness of the LED, and is an indication of ionization. 5. Decide whether each solute is polar, nonpolar, or polar covalent solute is soluble in water? Yes conducts electric current ? ( light is ON ) Yes Polar Ionic No Polar Covalent No Soluble in Hexane? Yes non-Polar Covalent No insoluble Part B: Effects of temperature on solubility of a gas Carbon dioxide is soluble in water, commonly found in soft drinks, seltzer water, beer, champagne, and the like. The gas forms a weak acid which gives these drinks a pleasant acidic taste, but the beverage “goes flat” after sitting in an unsealed container overnight. You will investigate the escape of gas which turns the acidic solution neutral. The reversible reaction: H2O + CO2 → H2CO3 → H+ + HCO3- → H2O + CO2↑ CO2 & water under pressure acidity via hydrogen ion CO2 evaporation 1. Fill one 150 or 250 mL beaker about 1/3 full with carbonated water (Perrier, seltzer, or equivalent). 2. Fill another with distilled water to the same level. Add enough bromphenol blue indicator to both to get a distinct color. 3. Place both beakers on a hot plate for about 10 minutes, or long enough to see a color change. Note the result in your lab report. Part C: Effects of temperature on solubility of a solid Most solids dissolve better and faster in hot versus cold water. You will observe this by adding a highly colored solute to cold and hot water to see the difference. 1. Place a large test tube 1/2 full of water in a beaker of hot water on a hot plate, and leave it to heat for about 5 minutes. Note temperature of the hot water bath 2. Place another large test tube 1/2 full of water in an ice-bath beaker and leave for about 5 minutes to cool to the ice temperature. Note temperature of the ice water bath 3. While one student holds the hot and cold water test tubes, a lab partner will drop equal amounts of Potassium Permanganate (about the size of a rice grain) into each tube simultaneously; and both will observe the speed and depth of color indicating degree of dissolution over time. Suggested observations at 10 seconds, 1 minute, and 5 minutes. Place the test tubes in a rack or beaker for 15 minutes and observe at room temperature. Is there any diffeence? DATA SHEETS SOLUBILITY & CONDUCTIVITY, Data Page 1 You MUST use DISTILLED WATER in theis experiment, tap water is conductive by itself. Samples of 12 materials in test tubes with water … which ones dissolved? (yes or no) Insert conductivity probe in water+solute test tubes, note LED brightness, or no light at all Repeat with samples of 11 materials in Hexane, which ones dissolved (yes or no) 1 Sucrose (table sugar) 2 Iodine 4 Calcium Chloride CaCl2 5 Sodium Chloride NaCl 6 Potassium Permanganate KMnO4 7 Cholesterol C27H46O 8 Ascorbic Acid (vitamin C) C6H8O6 9 Octanol C8H17OH 12 Hexane Solubility in Water (polar) Brightness Hexane (nonpolar) I2 NH4Cl 11 Vitamin E LED C12H22O11 3 Ammonium Chloride 10 Vegetable Oil Solubility in triglyceride α-Tocopherol C6H14 xxx (same thing) Notes to Conductivity Testing LED intensity indicates degree of ionic conductivity, bright light (≈20ma) means good conductor Dim light indicates reduction in electrical conductivity, fewer ions available, but still ionic. NO light indicates material is NON-conductive and NON-ionic Touching the leads together is useful to verify opertion and establish relative brightness OBSERVATIONS, data page 2 Part B: Temperature Effect on the Solubility of Gases Describe any color changes observed in the soda water plus indicator "Bromthymol Blue"after heating for about 20 minutes. Carbontion mkes the water acidic Solution color at room temperature (carbonated) Solution color after heating (no carbonation) Part C: Temperature Effect on the Solubility of Solids Place a test tube half full of distilled water in beaker of ice water for about 5 minutes, and do the same thing with a test tube immersed in hot water. Record the temperature in each beaker. Place several grains of Potassium Permnganate on tips of two wooden stir sticks. While one student holds the hot and cold water test tubes the lab partner will drop the permanganate in both test tubes at the same time. Observe the color difference for a few minutes, and place the test tubes aside (in a rack or beaker) until they come to room temperature (about 15 minutes). Observe the following, and describe intensity of color seen. Describe observations such as "light, dark, darker, darkest ... etc.) Cold Water test tube Hot Water test tube Temperature reading, oC The first few seconds after 30 seconds after 1 minute after sitting for 15 minutes. Summarize your observations on speed of solution versus temperature POST LAB QUESTIONS, data page 3 1 Based on YOUR solubility and conductivity results, describe differenced between Polar & non polar compounds in your own words. 2 For the 12 solutes you tested, classify each as ionic & polar, covalent & polar, or covalent & non polar. Note that if the material dissolved in water it must be polar, and if conducts an electric current it must be ionic as well. If a material dissolves in water but does NOT conduct an electric current, it must be polar-covalent ... only one of those in this experiment. ONLY ONE "YES"ANSWER PER ROW REQUIRED Solubility Criteria : Electrical Conductivity Criteria: 1 Sucrose C12H22O11 2 Iodine I2 3 Ammonium Chloride NH4Cl 4 Calcium Chloride CaCl2 5 Sodium Chloride NaCl 6 Potassium Permanganate KMnO4 7 Cholesterol 8 Ascorbic Acid (vitamin C) C6H8O6 9 Octanol 10 Vegetable Oil 11 Vitamin E 12 Hexane (a solvent) C27H46O C8H17OH triglyceride α-Tocopherol C6H14 IONIC Covalent COVALENT Polar water soluble conductive Polar water soluble non-conductive non-polar oil soluble non-conductive POST LAB QUESTIONS data page 4 3a What property do covalent solutes have in common regarding conductivity in water? 3b Did all covalent compounds we evaluated dissolve in Hexane? 4 Vitamins are classified as water soluble or fat soluble How would you classify the two vitamins you tested in terms of POLARITY of the solute? 4a Vitamin C 4b Vitamin A 5 Acetic acid (vinegar) is a covalent compound, but when dissolved in water, it is a weak electrolyte. How would you explain this result? 6 What change of the indicator in carbonated water did you observe after heating? 6a Describe color change 6b Eaplain the result: