File - RCPath study group
advertisement

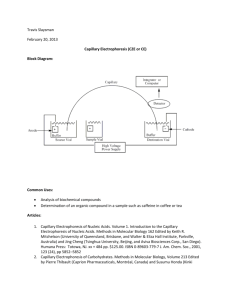
Outline the principles of electrophoresis and review the advantages and disadvantages of conventional electrophoresis and capillary zone electrophoresis giving examples of their use. Definition Electrophoresis is a term, which describes movement of charged particles under the influence of an electric field. Conventional zone electrophoresis describes movement of charged macromolecules in a porous support medium under the influence of current. Basic components and principles of conventional electrophoresis: The main components of conventional electrophoresis include the following: 1.Power supply, which gives the electromotive force 2.Support medium filled with a buffer solution: the support medium is usually porous with pores of different sizes (to allow molecules of certain sizes to migrate through) and neutral (to prevent electroendosmosis, a phenomenon which will be discussed later). The buffer serves two functions; it conveys the current and helps maintain a specific pH so analytes will have a specific constant charge during electrophoresis, depending on their isoelectric point. 3.The sample: it is applied into the support medium using a template with wells. 4.Detection system: when a sample is applied and after current application, different molecules migrate at different rates under the influence of current. At the end of the run, molecules (depending on their relative rates of migration) are separated into zones, which are fixed, stained and then quantified (or more accurately semi quantified) by a densitometer. Determinants of the rate of migration: Each charged particle should migrate towards the electrode of the opposite charge and the rate of migration depends on many factors including its size and shape, physical and chemical characteristics of the support medium, the strength of the electromotive force and the ionic strength of the buffer. The power supply plays an important role as it could be adjusted to give either a constant voltage or a constant current. Constant voltage power supply is effective only for shorter periods of electrophoresis. The movement of current in a resistive medium tend to produce heat, which is increased with time. Heat causes evaporation of the buffer thus increasing its ionic strength and subsequently reducing resistance. This result in acceleration of movements of particles and because of the increased in buffer ionic strength, the ionic cloud around the migration particles increases. This helps produce sharp zones on one hand. On the other hand, if heat generation is not controlled, this may end up in denaturation of heat labile proteins. So, constant current is preferred with longer electrophoresis times. Electroendosmosis: This refers to movement of buffer ions and solvent relative to the fixed support .It occurs when the support medium contains charged groups, which adsorbs negatively charged hydroxyl groups (of water) creating fixed positively charged buffer ions around them. When current is applied, the positively charged ions will migrate to the cathode and since they are hydrated, will result in net movement of the solvent toward the cathode. This will hinder the migration of negatively charged ions from the other direction resulting in their migration being stopped or even they are swept back into the other direction. This phenomenon is prevented by using neutral mediums like pure agarose and polyacrylamide gel. Capillary Zone Electrophoresis (CZE): This technique utilizes fused silica capillaries with small diameters as a medium for electrophoresis. The capillary is filled with buffer. Here, the electro-osmotic force EOF (due to electroendosmotic effect of the silica medium) is used to drive movement of charged particles. Since the EOF and the charge drive positively charged ions toward the cathode, they tend to migrate faster (the smaller molecules before the larger) leaving neutral molecules behind them followed by negatively charged ones. Molecules are being detected as they emerge from the other end of the capillary through a window made specifically for detection. Many methods are being used for detection including UV detection, laser induced fluorescence (LIF), electrochemical detection and even MS. CZE offers the advantage of heat dissipation due to its larger surface area to volume ratio compared to conventional electrophoresis. This allows the use of high voltages (in the range of kilovolts) so reducing the amount of time required for analysis to minutes. Small sample volumes (micrometers) are required. Moreover, resolution is improved by minimizing band broadening. Detection is also improved using this technique as it helps giving quantitative values. Limits of detection are decreased using more sensitive detectors especially LIF and MS.CZE is more amenable to automation compared with conventional electrophoresis. Applications of electrophoresis in the clinical lab: Both conventional and CZE are used in the lab for separation and quantification of charged particles including proteins. Conventional electrophoresis is widely used for serum protein electrophoresis (detection of monoclonal paraproteins), lipid electrophoresis, separation of isoenzymes, identification of haemoglobin variants and in molecular genetics techniques including separation and determination of the size of oligonucleotides produced by PCR techniques. CZE is also used for the same purposes as well as for separation, quantification and even elucidation of the molecular structure of organic ions, porphyrins, small molecules like drugs and aminoacids. So it has wider applications and offers the advantages mentioned above including amenability to automation, small sample requirement, faster, more sensitive and gives more accurate identification and quantification of molecules.