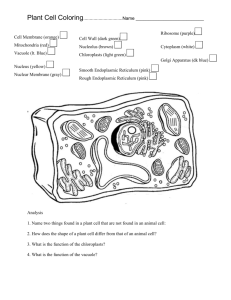
Madhya Pradesh Bhoj Open University
advertisement

SELF INSTRUCTIONAL MATERIAL M.Sc. PREVIOUS (BOTANY) PAPER –I CELL & MOLECULAR BIOLOGY OF PLANTS BLOCK -3 UNIT – 4 UNIT - 5 MADHYA PRADESH BHOJ ( OPEN) UNIVERSITY, BHOPAL 1 UNIT- IV Protein sorting , Cell shape and motility 4.0 Introduction of protein sorting 4.1 Objective 4.2 Protein translocation 4.3 Cell shape Cytoskeleton 4.4 Flagella and motility 1.4.1 Flagellar ultra-structure 1.4.2 Mechanism of flagellar movement 4.5 Cell cycle and Apoptosis 4.6 Role of Cyclins and CDK 4.7 Retinoblastoma and E2F Protein 4.8 Cytokinesis 4.9 Cell plate formation 4.10 Programmed cell death 4.11 Let us sum up 4.12 Assignment/ Activity 4.13 References 2 4.0 Introduction of protein sorting Protein targeting or protein sorting is the mechanism by which a cell transports proteins to the appropriate positions in the cell or outside of it. Sorting targets can be the inner space of an organelle, any of several interior membranes, the cell's outer membrane, or its exterior via secretion. This delivery process is carried out based on information contained in the protein itself. Correct sorting is crucial for the cell; errors can lead to diseases. Targeting signals are the pieces of information that enable the cellular transport machinery to correctly position a protein inside or outside the cell. This information is contained in the polypeptide chain or in the folded protein. The continuous stretch of amino acid residues in the chain that enables targeting are called signal peptides or targeting peptides. There are two types of targeting peptides, the pre-sequences and the internal targeting peptides. The presequences of the targeting peptide are often found at the N-terminal extension and is composed of between 6-136 basic and hydrophobic amino acids. In case of peroxisomes the targeting sequence is on the C-terminal extension mostly. Other signals are composed by parts which are separate in the primary sequence. To function these components have to come together on the protein surface by folding. They are called signal patches. In addition, protein modifications like glycosylation can induce targeting. 4.1 Objective 1. This unit focus on molecular control of the cell cycle in higher plants and do not deal with the developmental and environmental control of cell division. 2. 4.2 This unit emphasizes on the importance of protein targeting. Protein translocation In 1970, Günter Blobel conducted experiments on the translocation of proteins across membranes. He was awarded the 1999 Nobel prize for his findings. He discovered that many proteins have a signal sequence, that is, a short amino acid sequence at one end that functions like a postal code for the target organelle. The translation of mRNA into protein by a ribosome takes place within the cytosol. If the synthesized proteins "belong" in a different organelle, they can be transported there in either of two ways, depending on the protein. 3 Co-translational translocation The N-terminal signal sequence of the protein is recognized by a signal recognition particle (SRP) while the protein is still being synthesized on the ribosome. The synthesis pauses while the ribosome-protein complex is transferred to an SRP receptor on the endoplasmic reticulum (ER), a membrane-enclosed organelle. There, the nascent protein is inserted into the translocation complex that passes through the ER membrane. The signal sequence is immediately cleaved from the polypeptide once it has been translocated into the ER by signal peptidase in secretory proteins. This signal sequence processing differs for some ER transmembrane proteins. Within the ER, the protein is first covered by another protein to protect it from the high concentration of other proteins in the ER, giving it time to fold correctly. Once folded, the protein is modified as needed (for example, by glycosylation), then transported to the Golgi apparatus for further processing and goes to its target organelles or is retained in the ER by various ER retention mechanisms. Post translational translocation Even though most proteins are co-translationally translocated, some are translated in the cytosol and later transported to their destination. This occurs for proteins that go to a mitochondrion, a chloroplast, or a peroxisome (proteins that go to the latter have their signal sequence at the C terminus). Also, proteins targeted for the nucleus are translocated posttranslation. They pass through the nuclear envelope via nuclear pores. Sorting of proteins to mitochondria Most mitochondrial proteins are synthesized as cytosolic precursors containing uptake peptide signals. The pre-protein with pre-sequence targeted for the mitochondria is bound by receptors and the General Import Pore (GIP) (Receptors and GIP are collectively known as Translocase of Outer Membrane or TOM) at the outer membrane. The pre-protein is translocated through TOM as hairpin loops. The pre-protein is transported through the intermembrane space by small TIMs (which also acts as molecular chaperones) to the TIM23 or 22 (Translocase of Inner Membrane) at the inner membrane. Within the matrix the targeting sequence is cleaved off by mtHsp70. Three mitochondrial outer membrane receptors are known: TOM20, TOM22 and TOM70 The pre-sequence translocase23 (TIM23) is localized to the mitochondrial inner membrane and acts a pore forming protein which binds precursor proteins with its N-terminal. TIM23 4 acts a translocator for pre-proteins for the mitochondrial matrix, the inner mitochondrial membrane as well as for the intermembrane space. TIM50 is bound to TIM23 at the inner mitochondrial site and found to bind pre-sequences. Mitochondrial matrix targeting sequences are rich in positively charged amino acids and hydroxylated ones. Proteins are targeted to sub-mitochondrial compartments by multiple signals and several pathways. Targeting to the outer membrane, intermembrane space, and inner membrane often requires another signal sequence in addition to the matrix targeting sequence. Sorting of proteins to chloroplasts The pre-protein for chloroplasts contain a stromal import sequence or a stromal and thylakoid targeting sequence. The majority of pre-proteins are translocated through the Toc and Tic complexes located within the chloroplast envelope. In the stroma the stromal import sequence is cleaved off and intra-chloroplast sorting and folding continues. Sorting of proteins to both chloroplasts and mitochondria Many proteins are needed in both mitochondria and chloroplasts. In general the targeting peptide is of intermediate character to the two specific ones. The targeting peptides of these proteins have a high content of basic and hydrophobic amino acids, a low content of negatively charged amino acids. They have a lower content of alanine and a higher content of leucine and phenylalanine. The dual targeted proteins have a more hydrophobic targeting peptide than both mitochondrial and chloroplastic ones. Protein destruction Defective proteins are occasionally produced, or they may be damaged later, for example, by oxidative stress. Damaged proteins can be recycled. Proteins can have very different half life, mainly depending on their N-terminal amino acid residue. The recycling mechanism is mediated by ubiquitin. 4.3 Cell shape The Cytoskeleton. 5 One distinguishing feature that separates the eukaryotes from prokaryotes is the presence of the cytoskeleton in the former, and apparent absence of it in the latter. This suggests that the cytoskeleton was an important factor in eukaryote evolution. The function of the cytoskeleton is to maintain cellular shape, and is involved in intracellular organization, cell polarity, cell adhesion, and in some cases, motility. The cytoskeleton is composed of several different protein components. There are three general classes of cytoskeletal fibers: (1) microtubules, (2) intermediate filaments, and (3) actin filaments. a) Microtubules Compared to the other cytoskeletal fibers, the microtubule is rather large (15 to 35 nm diameter). Microtubules are composed of a globular protein, tubulin. The tubulin subunit is a heterodimer of alpha- and beta-tubulin . The microtubule itself is made up of 13 "protofilaments", which are each composed of alternating alpha and beta subunits. These protofilaments are cylindrically arranged to form a hollow tube. It is the arrangement of proto-filaments that makes up the microtubule. Microtubules are polar molecules i.e. they have a fast growing "plus end" and a slow growing "minus end". These strands are in a constant state of flux, termed "dynamic instability" (i.e. they continuously grow and fall apart). Free GTP (guanosine triphosphate) binds to the beta-tubulin, which alters its protein structure and enables it to join to the growing end of the strand. Then, there is a delayed GTP hydrolysis reaction to yield GDP (guanosine diphosphate). Thus, at the terminus of the filament a GTP cap is present (i.e. tubulin subunits are bound to GTP), while further down the strand, tubulin subunits are bound to GDP. The presence of GDP creates a weak bond between tubulin subunits, and therefore the filament is more likely to de-polymerize. In other words, if all the GTP is hydrolyzed in the filament, the filament is susceptible to falling apart (i.e. under a microscope, the microtubules are observed to shrink or even disappear). There are various proteins that are able to stabilize the microtubule, preventing de-polymerization these are known as "cap-proteins" or MAPs (Microtubule-Associated Proteins). There are also various other chemicals that can either stabilize or destabilize microtubules 6 Another group of cytoskeletal proteins are the intermediate filaments (IFs). IFs have an intermediate size between microtubules and actin filaments (7 to 10 nm diameter). There are, however, two general types of IFs: (1) cytoplasmic IFs, and (2) nuclear lamina. Cytoplasmic IFs are for mechanical stress and cell-to-cell junctions. Nuclear lamina create a meshwork beneath the inner nuclear membrane. One important difference to note between IF proteins and proteins for microtubules or for microfilaments, is that most IF protein subunits are filamentous, rather than globular (i.e. tubulin and actin subunits are globular). b) Microfilaments Actin filaments (or "microfilaments" these terms are used interchangeably) are the smallest of the cytoskeletal fibers (3 to 6 nm diameter). Microfilaments are flexible doubled-stranded fibers composed of polymers of the protein actin (contrast this structure to microtubules, which are hollow tubes composed of 13 protofilaments). Actin is present in all eukaryotes, and microfilaments are typically found in the cell cortex (i.e. just beneath the cell membrane). The actin subunit is globular and has a molecular mass of 43 kDa. As with microtubules, actin filaments are also dynamic and polar molecules (i.e. they have a fast growing "plus end" and a slow growing "minus end"). Free actin binds ATP (adenosine-triphosphate, as opposed to GTP in tubulin subunits) which enables polymerization, while ATP hydrolysis to ADP favours depolymerization. Unlike microtubules which undergo dynamic instability, actin filaments may use a different dynamic process. 7 Actin Binding Proteins. The actin-based cytoskeleton functions for bearing of tension and for compression resistance (i.e. it is like a shock suspension system, which gives mechanical strength and maintains structural integrity of a cell). In fact, there are different microfilament arrangements for a variety of functional purposes. There are, however, two general classes of microfilament arrangement: (1) bundles (parallel and contractile), and (2) gel-like networks. Different proteins (i.e. actin-binding proteins) mediate the different arrangements. Parallel bundles are structures where microfilaments are oriented with the same polarity (i.e. plus ends are all "pointed" in the same direction) and which are closely spaced. 8 4.4 Flagella and Motility Most motile move by use of flagella (flagellum) thread like locomotor appendages extending outward from the plasma membrane and cell wall. They are slender, rigid structures, about 20 nm across and up to 15 or 20 pm long. Flagella are so thin they cannot be observed directly with a bright-field microscope, but must be stained with special techniques designed to increase their thickness. The detailed structure of a flagellum can only be seen in the electron microscope. Bacterial species often differ distinctively in their patterns of flagella distribution. Monotrichous bacteria (trichous means hair) have one flagellum; if it is located at an end, it is said to be a polar flagellum. Amphitrichous bacteria (amphi mean "on both sides") have a single flagellum at each pole. In contrast, lophotrichous bacteria (lopho means tuft) have a cluster of flagella at one or both ends. Flagella are spread fairly evenly over the whole surface of peritrichours (peri means "around") bacteria. Flagellation patterns are very useful in identifying bacteria. 4.4.1 Flagellar Ultra structure : Transmission electron microscope studies have shown that the bacterial flagellum is composed of three parts. (1) The longest and most obvious portion is the filament, which extends from the cell surface to the tip. (2) A basal body is embedded in the cell; and (3) a 9 short, curved segment, the hook, links the filament to its basal body and acts as a flexible coupling. The filament is a hollow, rigid cylinder constructed of a single protein celled flagellin, which ranges in molecular weight from 30,000 to 60,000. The filament ends with a capping protein. Some bacteria have sheaths surrounding their flagella. For example Vibrio cholerae has a lipopolysaccharide sheath. The hook and basal body are quite different from the filament of different protein subunits. The basal body is the most complex part of a flagellum. In E.coli and most gram-negative bacteria, the body has four rings connected to a central rod. The outer L and P rings associate with the lipopolysaccharide and peptidoglycan layers, respectively. The inner M ring contacts the plasma membrane. Gram-positive bacteria have only two basal body rings, an inner ring connected to the plasma membrane and an outer one probably attached to the peptidoglycan. Flagellar Synthesis The synthesis of flagella is a complex process involving at least 20 to 30 genes. Besides the gene for flagellin, 10 or more genes code for hook and basal body proteins ; other genes are concerned with the control of flagellar construction or function. It is not known how the cell regulates or determines the exact location of flagella. 4.4.2 The Mechanism of Flagellar Movement Procaryotic flagella operate differently from eukaryotic flagella. The filament is in the shape of a rigid helix, and the bacterium moves when this helix rotates. Considerable evidence shows that flagella act just like propellers on a boat. Bacterial mutants with straight flagella or abnormally long hook regions (polyhook mutants) cannot swim. When bacteria are tethered to a glass slide using antibodies to filament or hook proteins, the cell body rotates rapidly about the stationary flagellum. If polystyrene-latex beads are attached to flagella, the beads spin about the flagellar axis due to flagellar rotation. The flagellar motor can rotate very rapidly. The E. coli motor rotates 270 revolutions per second ; Vibrio alginolyticus averages 1,100 rps. 10 The direction of flagellar rotation determines the nature of bacterial movement. Monotrichous, polar flagella rotate slowly clockwise. The rotating helical flagellar filament thrusts the cell forward in a run with the flagellum trailing behind. Monotrichous bacteria stop and tumble randomly by reversing the direction of flagellar rotation. Peritrichously flagellated bacteria operate in a somewhat similar way to move forward, the flagella rotate counterclockwise. As they do so, they bend at their hooks to form a rotating bundle that propels them forward. Clockwise rotation of the flagella disrupts the bundle and the cell tumbles. Because bacteria swim though rotation of their rigid flagella, there must be some sort of motor at the base. A rod or shaft extends from the hook and ends in the M ring, which can rotate freely in the plasma membrane. It is believed that the S ring is attached to the cell wall in gram positive cells and does not rotate . The P and L rings of gram negative bacteria would act as bearings for the rotating rod. There is some evidence that the basal body is a passive structure and rotates within a membrane embedded protein. The relationship of flagellar rotation to bacterial movement. The rotor is like an electrical motor turns in the center of a ring of electromagnets (the stator). 11 The exact mechanism that drives basal drives basal body rotation still is not clear provides a more detailed depiction of the basal body in gram negative bacteria. The rotor portion of the motor seems to be made primarily of a rod, the M ring, and C ring joined to it on the cytoplasmic side of the basal body. These two rings are made of several proteins. The two most important proteins in the stator part of the motor are Mot A and Mot B. These form a proton channel thought the plasma membrane, and Mot B also anchors the Mot complex to cell wall peptidoglycan. There is some evidence that Mot A and G directly interact during flagellar rotation. This rotation is driven by proton or sodium gradients in prokaryotes, not directly by ATP as is the case with eukaryotic flagella. Bacteria can move by mechanisms other than falgellar rotations. Spriochetes are helical bacteria that travel through viscous substances such as mucus or mud by flexing and spinning movements caused by a special axial filament composed of periplasmic flagella. A very different type of motility, gliding motility, is employed by many bacteria : cyanobacteria . 4.5 Cell cycle and apoptosis The cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell leading to its replication. These events can be divided in two brief periods: interphase during which the cell grows, accumulating nutrients needed for mitosis and duplicating its DNA and the mitotic (M) phase, during which the cell splits itself into two distinct cells, often called "daughter cells". The cell-division cycle is a vital process by which a single-celled fertilized egg develops into a mature organism, as well as the process by which hair, skin, blood cells, and some internal organs are renewed. Phases of the cell cycle The cell cycle consists of four distinct phases: G1 phase, S phase, G2 phase (collectively known as interphase) and M phase. M phase is itself composed of two tightly coupled 12 processes: mitosis, in which the cell's chromosomes are divided between the two daughter cells, and cytokinesis, in which the cell's cytoplasm divides forming distinct cells. Activation of each phase is dependent on the proper progression and completion of the previous one. Cells that have temporarily or reversibly stopped dividing are said to have entered a state of quiescence called G0 phase. M phase The relatively brief M phase consists of nuclear division (karyokinesis) and cytoplasmic division (cytokinesis). In plants and algae, cytokinesis is accompanied by the formation of a new cell wall. The M phase has been broken down into several distinct phases, sequentially known as prophase, Prometaphase, metaphase, anaphase and telophase leading to cytokinesis. Interphase After M phase, the daughter cells each begin interphase of a new cycle. Although the various stages of interphase are not usually morphologically distinguishable, each phase of the cell cycle has a distinct set of specialized biochemical processes that prepare the cell for initiation of cell division. G1 phase The first phase within interphase, from the end of the previous M phase till the beginning of DNA synthesis is called G1 (G indicating gap or growth). During this phase the biosynthetic activities of the cell, which had been considerably slowed down during M phase, resume at a high rate. This phase is marked by synthesis of various enzymes that are required in S phase, mainly those needed for DNA replication. Duration of G1 is highly variable, even among different cells of the same species. S phase The S phase starts when DNA synthesis commences; when it is complete, all of the chromosomes have been replicated, i.e., each chromosome has two (sister) chromatids. Thus, during this phase, the amount of DNA in the cell has effectively doubled, though the ploidy of the cell remains the same. Rates of RNA transcription and protein synthesis are very low during this phase. An exception to this is histone production, most of which occurs during the S phase. The duration of S phase is relatively constant among cells of the same species. 13 G2 phase The cell then enters the G2 phase, which lasts until the cell enters mitosis. Again, significant protein synthesis occurs during this phase, mainly involving the production of microtubules, which are required during the process of mitosis. Inhibition of protein synthesis during G2 phase prevents the cell from undergoing mitosis. G0 phase The term "post-mitotic" is sometimes used to refer to both quiescent and senescent cells. Non-proliferative cells in multicellular eukaryotes generally enter the quiescent G0 state from G1 and may remain quiescent for long periods of time, possibly indefinitely (as is often the case for neurons). This is very common for cells that are fully differentiated. Cellular senescence is a state that occurs in response to DNA damage or degradation that would make a cell's progeny nonviable; it is often a biochemical alternative to the self-destruction of such a damaged cell by apoptosis. Cell cycle inhibitors Two families of genes, the cip/kip family and the INK4a/ARF (Inhibitor of Kinase 4/Alternative Reading Frame) prevent the progression of the cell cycle. Because these genes are instrumental in prevention of tumor formation, they are known as tumor suppressors. The cip/kip family includes the genes p21, p27 and p57. They halt cell cycle in G1 phase, by binding to, and inactivating, cyclin-CDK complexes. p21 is activated by p53 (which in turn is triggered by DNA damage e.g. due to radiation). p27 is activated by Transforming Growth Factor β (TGF β), a growth inhibitor. The INK4a/ARF family includes p16INK4a, which binds to CDK4 and arrests the cell cycle in G1 phase, and p14arf which prevents p53 degradation. And the amount of chromosomes are able to double at the same rate as in phase 2 Checkpoints Cell cycle checkpoints are used by the cell to monitor and regulate the progress of the cell cycle. Checkpoints prevent cell cycle progression at specific points, allowing verification of necessary phase processes and repair of DNA damage. The cell cannot proceed to the next phase until checkpoint requirements have been met. 14 Several checkpoints are designed to ensure that damaged or incomplete DNA is not passed on to daughter cells. Two main checkpoints exist: the G1/S checkpoint and the G2/M checkpoint. G1/S transition is a rate-limiting step in the cell cycle and is also known as restriction point. An alternative model of the cell cycle response to DNA damage has also been proposed, known as the post replication checkpoint. p53 plays an important role in triggering the control mechanisms at both G1/S and G2/M checkpoints. The development of the term apoptosis Already since the mid-nineteenth century, many observations have indicated that cell death plays a considerable role during physiological processes of multicellular organisms, particularly during embryogenesis and metamorphosis . The term programmed cell death was introduced in 1964, proposing that cell death during development is not of accidental nature but follows a sequence of controlled steps leading to locally and temporally defined selfdestruction . Eventually, the term apoptosis had been coined in order to describe the morphological processes leading to controlled cellular self-destruction and was first introduced in a publication by Kerr, Wyllie and Currie [Kerr, 1972]. Apoptosis is of Greek origin, having the meaning "falling off or dropping off", in analogy to leaves falling off trees or petals dropping off flowers. This analogy emphasizes that the death of living matter is an integral and necessary part of the life cycle of organisms. The apoptotic mode of cell death is an active and defined process which plays an important role in the development of multicellular organisms and in the regulation and maintenance of the cell populations in tissues upon physiological and pathological conditions. It should be stressed that apoptosis is a welldefined and possibly the most frequent form of programmed cell death, but that other, nonapoptotic types of cell death also might be of biological significance . The significance of apoptosis The development and maintenance of multicellular biological systems depends on a sophisticated interplay between the cells forming the organism, it sometimes even seems to involve an altruistic behaviour of individual cells in favour of the organism as a whole. During development many cells are produced in excess which eventually undergo programmed cell death and thereby contribute to sculpturing many organs and tissues . Taken together, apoptotic processes are of widespread biological significance, being involved in e.g. development, differentiation, proliferation/homoeostasis, regulation and function of 15 the immune system and in the removal of defect and therefore harmful cells. Thus, dysfunction or deregulation of the apoptotic program is implicated in a variety of pathological conditions. Defects in apoptosis can result in cancer, autoimmune diseases and spreading of viral infections, while neurodegenerative disorders and AIDS diseases are caused or enhanced by excessive apoptosis. Due to its importance in such various biological processes, programmed cell death is a widespread phenomenon, occurring in all kinds of metazoans such as in mammals, insects, nematodes. Moreover, programmed cell death also might play a role in plant biology, and apoptosis-like cell death mechanisms even have been observed and used as a model system in yeast. Fascinating insights into the origin and evolution of programmed cell death might possibly be given by the fact, that programmed cell death is also an integral part of the life cycle of other unicellular eukaryotes and that even prokaryotes. Morphological features of apoptosis Apoptotic cells can be recognized by stereotypical morphological changes: the cell shrinks, shows deformation and looses contact to its neighboring cells. Its chromatin condenses and migrate at the nuclear membrane and finally the cell is fragmented into compact membraneenclosed structures, called 'apoptotic bodies' which contain cytosol, the condensed chromatin, and organelles. The apoptotic bodies are engulfed by macrophages and thus are removed from the tissue without causing an inflammatory response. Those morphological changes are a consequence of characteristic molecular and biochemical events occurring in an apoptotic cell, most notably the activation of proteolytic enzymes which eventually mediate the cleavage of DNA into oligo-nucleosomal fragments as well as the cleavage of a multitude of specific protein substrates which usually determine the integrity and shape of the cytoplasm or organelles . 4.6 Role of Cyclins and CDKs Two key classes of regulatory molecules, cyclins and cyclin-dependent kinases (CDKs), determine a cell's progress through the cell cycle. Leland H. Hartwell, R. Timothy Hunt, and Paul M. Nurse won the 2001 Nobel Prize in Physiology or Medicine for their discovery of these central molecules. Many of the genes encoding cyclins and CDKs are conserved among all eukaryotes, but in general more complex organisms have more elaborate cell cycle control systems that incorporate more individual components. Many of the relevant genes were first identified by studying yeast, especially Saccharomyces cerevisiae; genetic nomenclature in 16 yeast dubs many of these genes cdc (for "cell division cycle") followed by an identifying number, e.g., cdc25. Cyclins form the regulatory subunits and CDKs the catalytic subunits of an activated heterodimer; cyclins have no catalytic activity and CDKs are inactive in the absence of a partner cyclin. When activated by a bound cyclin, CDKs perform a common biochemical reaction called phosphorylation that activates or inactivates target proteins to orchestrate coordinated entry into the next phase of the cell cycle. Different cyclin-CDK combinations determine the downstream proteins targeted. CDKs are constitutively expressed in cells whereas cyclins are synthesised at specific stages of the cell cycle, in response to various molecular signals. Cyclin-dependent kinases (CDKs) are typical serine/threonine kinases that display the 11 subdomains shared by all kinases. Among the 19,099 predicted genes, there are 14 CDKs and 34 cyclins; nine CDKs and 11 cyclins have been identified in man, referred to as CDK1CDK9. This also allows the activating phosphorylation on Thr160 (by CDK7/cyclin H/MAT1). The second conformational change induced by cyclin binding is found within the ATP-binding site where a reorientation of the amino acid side chains induces the alignment of the triphosphate of ATP necessary for phosphate transfer. The strong sequence homology between the catalytic domains of different CDKs suggests that their tridimensional structures will be similar. Progression through the G1, S, G2 and M phases of the cell division cycle is directly controlled by CDKs. In early-mid G1, extracellular signals modulate the activation of CDK4 and CDK6 associated with D-type cyclins. These complexes phosphorylate and inactivate the retinoblastoma protein pRb, resulting in the release of the E2F and DP1 transcription factors which control the expression of genes required for the G1/S transition and S phase progression. The CDK2/cyclin E complex, which is responsible for the G1/S transition, also regulates centrosome duplication. During S phase, CDK2/cyclin A phosphorylates different substrates allowing DNA replication and the inactivation of G1 transcription factors. Around the S/G2 transition, CDK1 associates with cyclin A. Later, CDK1/cyclin B appears and triggers the G2/M transition by phosphorylating a large set of substrates. Phosphorylation of the anaphase promoting complex (APC) by CDK1/cyclin B is required for transition to anaphase and completion of mitosis General mechanism of cyclin-CDK interaction 17 Upon receiving a pro-mitotic extracellular signal, G1 cyclin-CDK complexes become active to prepare the cell for S phase, promoting the expression of transcription factors that in turn promote the expression of S cyclins and of enzymes required for DNA replication. The G1 cyclin-CDK complexes also promote the degradation of molecules that function as S phase inhibitors by targeting them for ubiquitination. Once a protein has been ubiquitinated, it is targeted for proteolytic degradation by the proteasome. Active S cyclin-CDK complexes phosphorylate proteins that make up the pre-replication complexes assembled during G1 phase on DNA replication origins. The phosphorylation serves two purposes: to activate each already-assembled pre-replication complex, and to prevent new complexes from forming. This ensures that every portion of the cell's genome will be replicated once and only once. The reason for prevention of gaps in replication is fairly clear, because daughter cells that are missing all or part of crucial genes will die. However, for reasons related to gene copy number effects, possession of extra copies of certain genes would also prove deleterious to the daughter cells. Mitotic cyclin-CDK complexes, which are synthesized but inactivated during S and G2 phases, promote the initiation of mitosis by stimulating downstream proteins involved in chromosome condensation and mitotic spindle assembly. A critical complex activated during this process is a ubiquitin ligase known as the anaphase-promoting complex (APC), which promotes degradation of structural proteins associated with the chromosomal kinetophore. APC also targets the mitotic cyclins for degradation, ensuring that telophase and cytokinesis can proceed. Specific action of cyclin-CDK complexes Cyclin D is the first cyclin produced in the cell cycle, in response to extracellular signals (eg. growth factors). Cyclin D binds to existing CDK4, forming the active cyclin D-CDK4 complex. Cyclin D-CDK4 complex in turn phosphorylates the retinoblastoma susceptibility protein (RB). The hyperphosphorylated RB dissociates from the E2F/DP1/RB complex (which was bound to the E2F responsive genes, effectively "blocking" them from transcription), activating E2F. Activation of E2F results in transcription of various genes like cyclin E, cyclin A, DNA polymerase, thymidine kinase, etc. Cyclin E thus produced binds to CDK2, forming the cyclin E-CDK2 complex, which pushes the cell from G1 to S phase (G1/S transition). Cyclin A along with CDK2 forms the cyclin A-CDK2 complex, which initiates the G2/M transition. Cyclin B-CDK1 complex activation causes breakdown of nuclear 18 envelope and initiation of prophase, and subsequently, its deactivation causes the cell to exit mitosis. 4.7 Retinoblastoma and E2F Protein. Retinoblastoma is a human childhood disease, involving a tumor of the retina. It occurs both as a heritable trait and sporadically (by somatic mutation). It is often associated with deletions of band q 14 of human chromosome 13. The RB gene has been localized to this region by molecular cloning. Summarizes the situation. Retinoblastoma arises when both copies of the RB gene are in-parental chromosome carries an alteration in this region. A somatic event in retinal cells that causes loss of the other copy of the RB gene causes a tumor. In the sporadic form of the disease, the parental chromosomes are normal, and both RB alleles are lost by (individual) somatic events. The cause of retinoblastoma is therefore loss of protein function. Loss of RB is involved also in other forms of cancer, including osteosarcomas and small cell lung cancers. RB is a nuclear phosphoprotein that influences the cell cycle. In resting (G0/G1) cells, RB is not phosphorylated. RB is phoshorylated during the cell cycle by cyclin/cdk complexes, most particularly at the end of G1; it is dephosphorylated during mitosis. The non-phosphorylated form of RB specifically binds several protein, and these interactions therefore occur only during part of the cell cycle (prior to S phase). Phosphorylation releases these protein. The target proteins include the E2F group of transcription factors, which activate target genes whose products are essential for S phase. Binding to RB inhibits the ability of E2F to activate transcription, Which suggests that RB may repress the expression of genes dependent on E2F. In this way, RB indirectly prevents cells from entering S phase. Also, the RB-E2F complex directly represses some target genes, so its dissociation allows them to be expressed. Certain viral tumor antigens bind specifically to the non-phosphorylated from of RB. The best characterized are SV40 T antigen and adenovirus E1A. This suggests the model shown in Non-phosphorylated RB prevents cell proliferation , this activity must be suppressed in order to pass through the cell cycle, which is accomplished by the cyclicphosphorylation. And it may also be suppressed when a tumor antigen sequesters the nonphosphorylated bind E2F, the E2F is permanently free to allow entry into S phase (and the RB-E2F complex is not available to repress its target genes.) 19 Over-expression of RB impedes cell growth. An indication of the importance of RB for cell proliferation is given by the properties of an osteosarcoma cell line that lacks RB; when RB is introduced into this cell line, its growth is impeded. However, the inhibition can be overcome by expression of D cyclins, which form cdk-cyclin combinations that phosphorylate RB. RB is not the only proteins of its type : proteins with related sequences, called p107 and p130, have similar properties. The connection between the cell cycle and tumor genesis is illustrated in several regulators are identified as tumor suppressors by the occurrence of inactivating mutations in tumors. In addition to RB itself, there are the small inhibitory proteins (most notably p16 and possibly p21), and D cyclin. Although these proteins (most notable RB) play a role in the cycle of a proliferating cell, the role that is relevant for tumor-genesis is more probably their function in the quiescent (G0) state. In quiescent cells, RB is not phosphorylated, D cylin levels are low or absent, and p16, p21 p27 ensure inactivity of cdk-cyclin complexes, The loss of this circuit is necessary for unrestrained growth. Check your progress : 1 1. Notes- Write your answer in the space given. 2. Compare your answer with the one given at the end of the unit. 1. What are the general classes of cytoskeletal fibers? 2. What are the phases of cell cycle? 3. What is Apoptosis? 4. What is Anaphase-promoting complex (APC)? 5. What is Retinoblastoma? ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------20 4.8 Cytokinesis Mitosis is the process of separating the duplicates of each of the cell's chromosomes. It is usually followed by division of the cell. However, there are cases (cleavage in the insect embryo is an example) where the chromosomes undergo the mitotic process without division of the cell. Thus a special term, cytokinesis, for the separation of a cell into two. In animal cells, a belt of actin filaments forms around the perimeter of the cell, midway between the poles. The interaction of actin and a myosin (not the one found in skeletal muscle) tightens the belt, and the cell is pinched into two daughter cells. In plant cells, a cell plate forms where the metaphase plate had been. The cell plate is synthesized by the fusion of multiple membrane-bounded vesicles. Their fusion supplies new plasma membrane for each of the two daughter cells. Synthesis of a new cell wall between the daughter cells then occurs at the cell plate. 21 Cytokinesis is the process whereby the cytoplasm of a single eukaryotic cell is divided to form two daughter cells. It usually initiates during the late stages of mitosis, and sometimes meiosis, splitting a binucleate cell in two, to ensure that chromosome number is maintained from one generation to the next. In plant cells, a dividing structure known as the cell plate forms across the centre of the cytoplasm and a new cell wall forms between the two daughter cells. [1] 4.9 Cell plate formation Due to the presence of a cell wall, cytokinesis in plant cells is significantly different from that in animal cells. Rather than forming a contractile ring, plant cells construct a cell plate in the middle of the cell. The Golgi apparatus releases vesicles containing cell wall materials. These vesicles fuse at the equatorial plane and form a cell plate. The cell plate begins as a fusion tube network, which then becomes a tubule-vesicular network (TVN) as more components are added. The TVN develops into a tubular network, which then becomes a fenestrated sheet which adheres to the existing plasma membrane. Phragmoplast and cell plate formation in a plant cell during cytokinesis. Left side: Phragmoplast forms and cell plate starts to assemble in the center of the cell. Towards the right: Phragmoplast enlarges towards the outside of the cell, leaving behind mature cell plate in the center. The cell plate will transform into the new cell wall once cytokinesis is complete. Cytokinesis in terrestrial plants occurs by cell plate formation. This process entails the delivery of Golgi-derived and endosomal vesicles carrying cell wall and cell membrane components to the plane of cell division and the subsequent fusion of these vesicles within this plane. After formation of an early tubule-vesicular network at the center of the cell, the initially labile cell plate consolidates into a tubular network and eventually a fenestrated sheet. The cell plate grows outward from the center of the cell to the parental plasma membrane with which it will fuse, thus completing cell division. Formation and growth of the cell plate is dependent upon the phragmoplast, which is required for proper targeting of Golgi-derived vesicles to the cell plate. 22 As the cell plate matures in the central part of the cell, the phragmoplast disassembles in this region and new elements are added on its outside. This process leads to a steady expansion of the phragmoplast, and concomitantly, to a continuous retargeting of Golgi-derived vesicles to the growing edge of the cell plate. Once the cell plate reaches and fuses with the plasma membrane the phragmoplast disappears. This event not only marks the separation of the two daughter cells, but also initiates a range of biochemical modifications that transform the flexible cell plate into a cellulose-rich, stiff primary cell wall. The heavy dependence of cell plate formation on active Golgi stacks explains why plant cells, unlike mammalian cells, do not disassemble their secretion machinery during cell division. 4.10 Programmed cell death Programmed cell-death (PCD) is death of a cell in any form, mediated by an intracellular program. In contrast to necrosis, which is a form of cell-death that results from acute tissue injury and provokes an inflammatory response, PCD is carried out in a regulated process which generally confers advantage during an organism's life-cycle. PCD serves fundamental functions during both plant and metazoa (multicellular animals) tissue development. Apoptosis or Type I cell-death Autophagic or Type II cell-death ( cytoplasmic: characterized by the formation of large vacuoles which eat away organelles in a specific sequence prior to the nucleus being destroyed.) Besides these two types of PCD, other pathways have been discovered. Called "non-apoptotic programmed cell-death" (or "caspase-independent programmed cell-death" or "necrosis-like programmed cell-death") these alternative routes to death are as efficient as apoptosis and can function as either backup mechanisms or the main type of PCD. Plant cells undergo particular processes of PCD which are similar to autophagic cell death. However, some common features of PCD are highly conserved in both plants and metazoa. History The concept of "programmed cell-death" was used by Lockshin & Williams in 1964 in relation to insect tissue development, around eight years before "apoptosis" was coined. Since then, PCD has become the more general of these two terms. PCD has been the subject of increasing attention and research efforts. This trend has been highlighted with the award of 23 the 2002 Nobel Prize in Physiology or Medicine to Sydney Brenner (United Kingdom), H. Robert Horvitz (US) and John E. Sulston (UK). The cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell leading to its replication. In "APL regulates vascular tissue identity in Arabidopsis", Bonke and colleagues state that one of the two long-distance transport systems in vascular plants, xylem, consists of several cell-types "the differentiation of which involves deposition of elaborate cell-wall thickenings and programmed cell-death." The authors emphasize that the products of plant PCD play an important structural role. Basic morphological and biochemical features of PCD have been conserved in both plant and animal kingdoms. It should be noted, however, that specific types of plant cells carry out unique cell-death programs. These have common features with animal apoptosis for instance, nuclear DNA degradation but they also have their own peculiarities, such as nuclear degradation being triggered by the collapse of the vacuole in tracheids elements of the xylem. PCD in pollen prevents inbreeding 24 During pollination, plants enforce self-incompatibility (SI) as an important means to prevent self-fertilization. Research on the corn poppy (Papaver rhoeas) has revealed that proteins in the pistil on which the pollen lands, interact with pollen and trigger PCD in incompatible (i.e. self) pollen. The researchers, Steven G. Thomas and Veronica E. Franklin-Tong, also found that the response involves rapid inhibition of pollen-tube growth, followed by PCD. Check your progress :2 1.Notes- Write your answer in the space given. 2.Compare your answer with the one given at the end of the unit. 1. What is programmed cell death? 2. What is cytokinesis? -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------4.11 Let us sum up 1. Protein targeting or protein sorting is the mechanism by which a cell transports proteins to the appropriate positions in the cell or outside of it. Sorting targets can be the inner space of an organelle, any of several interior membranes, the cell's outer membrane, or its exterior via secretion. This delivery process is carried out based on information contained in the protein itself. Correct sorting is crucial for the cell; errors can lead to diseases. 2. The cytoskeleton is to maintain cellular shape, and is involved in intracellular organization, cell polarity, cell adhesion, and in some cases, motility. The cytoskeleton is composed of several different protein components. There are three general classes of cytoskeletal fibers: (1) microtubules, (2) intermediate filaments, and (3) actin filaments. 3. The cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell leading to its replication. These events can be divided in two brief periods: interphase during which the cell grows, accumulating nutrients needed for mitosis and duplicating its DNA and the mitotic (M) phase, during which the cell splits itself into two distinct cells, often called "daughter cells". The cell-division cycle is a vital process by which 25 a single-celled fertilized egg develops into a mature organism, as well as the process by which hair, skin, blood cells, and some internal organs are renewed. 4. Retinoblastoma is a human childhood disease, involving a tumor of the retina. It occurs both as a heritable trait and sporadically (by somatic mutation). It is often associated with deletions of band q 14 of human chromosome 13. The RB gene has been localized to this region by molecular cloning. 5. Cytokinesis is the process whereby the cytoplasm of a single eukaryotic cell is divided to form two daughter cells. It usually initiates during the late stages of mitosis, and sometimes meiosis, splitting a binucleate cell in two, to ensure that chromosome number is maintained from one generation to the next. 6. Programmed cell-death (PCD) is death of a cell in any form, mediated by an intracellular program. In contrast to necrosis, which is a form of cell-death that results from acute tissue injury and provokes an inflammatory response. Check your progress 1 : The key 1. Three general classes of cytoskeletal fibers: (1) microtubules, (2) intermediate filaments, and (3) actin filaments. 2. The cell cycle consists of four distinct phases: G1 phase, S phase, G2 phase and M phase. 3. The cell shrinks, shows deformation and looses contact to its neighboring cells. Its chromatin condenses and migrate at the nuclear membrane and finally the cell is fragmented into compact membrane-enclosed structures, called 'apoptotic bodies' which contain cytosol, the condensed chromatin, and organelles which is a form of cell death is called apoptosis. 4. A critical complex activated during Cyclin-CDK Complex this process is a ubiquitin ligase known as the anaphase-promoting complex (APC), which promotes degradation of structural proteins associated with the chromosomal kinetophore. 5. Retinoblastoma is a human childhood disease, involving a tumor of the retina. It occurs both as a heritable trait and sporadically (by somatic mutation). Check your progress 2 : The key 26 1. Programmed cell-death (PCD) is death of a cell in any form, mediated by an intracellular program. In contrast to necrosis, which is a form of cell-death that results from acute tissue injury and provokes an inflammatory response, PCD is carried out in a regulated process which generally confers advantage during an organism's life-cycle 2. Cytokinesis is the process whereby the cytoplasm of a single eukaryotic cell is divided to form two daughter cells 4.12 Assignment/ Activity 1. Study and explain the cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell. 2. Retinoblastoma is a human childhood disease, involving a tumor of the retina, collect some more information on it. 4.13 References 1. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. (2003). Molecular Biology of the Cell. Ch 17. Garland Science: New York. 4th ed 2. J. A. Smith and L. Martin (April 1, 1973). "Do Cells Cycle?". PNAS 70 (4): 12631267. doi:10.1073/pnas.70.4.1263. PMID 4515625. 3. Morgan DO. (2007) The Cell Cycle: Principles of Control. New Science Press: London. 4. R.S. Prather, A.C. Boquest, B.N. Day (1999). "Cell Cycle Analysis of Cultured Porcine Mammary Cells". Cloning 1 (1): 17-24. doi:10.1089/15204559950020067. PMID 16218827. 5. Stephen J. Elledge (6 December 1996). "Cell Cycle Checkpoints: Preventing an Identity Crisis". Science 274 (5293): 1664-1672. doi:10.1126/science.274.5293.1664. PMID 8939848 6. Seydou Samaké, Lawrence C. Smith (1997 Oct 15). "Synchronization of cell division in eight-cell bovine embryos produced in vitro: Effects of nocodazole". Theriogenology 48 (6): 969-76. doi:10.1016/S0093-691X(97)00323-3. PMID 16728186. 27 7. Zuzarte-Luis, V and Hurle, JM (2002). "Programmed cell death in the developing limb." Int J Dev Biol 46(7): 871-6. 28 UNIT- V OTHER CELLULAR ORGANELLES 5.0 Introduction 5.1 Objective 5.2 Peroxisomes 5.3 Golgi apparatus 5.4 Lysosomes 5.5 Endoplasmic reticulum 5.6 Techniques in cell biology 5.7 FISH 5.8 Confocal microscopy 5.9 Let us sum up 5.10 Assignment/ Activity 5.11 Reference 29 5.0 Introduction Microbodies : Structure and Types Microbodies are spherical or oblate in form. They are bounded by a single membrane and have an interior or matrix which is amorphous or granular Micrbodies are most easily distinguished from other cell organelles by their content of catalase enzyme. Catalase can be visualized with the electron microscope when cells are treated with the stain DAB (i.e., 3,3'-diaminobenzidine). The product is electron opaque and appears as dark regions in the cell where catalase is present The technique of isolation of microbodies of plant tissues includes the following steps : (1) Tissues are group very carefully to save microbodies from disruption. (2) The homogenate is with differential centrifugation to obtain a fraction of the cell homogenate which is rich in microbodies. (3) The enriched fraction is subjected to isopycnic ultra-centrifugation on discontinuous or continuous sucrose density gradient. Recent biochemical studies have distinguished two types of microbodies, namely peroxisomes and glycoxysomes. These two organelles differ both in their enzyme complement and in the type of tissue in which they are found. Peroxisomes are found in animal cells and the leaves of higher plants. They contain catalases and oxidases (e.g., D-amino oxidase and urate oxidase). In both they participate in the oxidation of substrates, producing hydrogen peroxide which is subsequently destroyed by catalase activity. In plant cells, peroxisomes remain associated with ER. chloroplasts and mitochondria and are involved in photorespiration. Gloxysomes occur only in plant cells and are particularly abundant in germinating seeds which store fats as a reserve food material. They contain enzymes of glyoxylate cycle besides the catalases and oxidases 5.1 Objectives 1. This unit will fulfill the basic introduction , function, origin and chemical composition of some organelles. 2. Students will find the text useful as it will help in understanding some molecular diagnostic technique. 5.2 Peroxisomes 30 Peroxisomes occur in many animal cells and in a wide range of plants. They are present in all photosynthetic cells of higher plants in etiolated leaf tissue, in coleoptiles and hypocotyls, in tobacco stem and callus, in ripening pear fruits and also in Euglenophyta, Protozoa, brown algae, fungi liverworts, mosses and ferns. Peroxisomes are variable in size and shape, but usually appear circular in cross section having diameter between 0.2 and 1.5m (0.2 and 0.25 m diameter in most mammalian tissues : 0.5m diameter in rat liver cells). They have a single limiting unit membrane of lipid and protein molecules, which encloses their granular matrix. In some cases (e.g., in the festuciod grasses) the matrix contains numerous threads or fibrils, while in others they are observed to contain either an amorphous nucleoid or a dense inner core which in many species shows a regular crystalloid structure (e.g., tobacco leaf cell, Newcomb and Frederick, 1971). Little is known about the function of the core, except that it is the site of the enzyme urate oxidase in rat liver peroxisomes and much of the catalase in some plants (see Hall et al., 1974). Recently, a possible relationship has been stressed between peroxides and free radicals (such as superoxide anion -O2-) with the process of aging. These radicals may act on DNA molecule to produce mutations altering the transcription into mRNA and the translation into proteins. In addition, free radicals and peroxides can affect the membranes by causing peroxidation of lipids and proteins. For these reasons reducing compounds such as vitamin E or enzymes such as superoxide dismutase could play a role in keeping the healthy state of a cell. Glycolate cycle- Peroxisomes of plant leaves contain catalaze together with the enzymes of glycolate pathway, as glycolate oxidase, glutamate glyoxylate, serine-glyoxylate and aspirate--ketoglutarate aminotransferases, hydroxyl pyruvate reductase and malic dehydrogenase. They also contain FAD, NAD and NADP coenzymes. The glycolate cycle is throught to bring about the formation of the amino acids-glycine and serine-from the non-phosphorylated intermediates of phostosynthetic carbon reduction cycle, i.e., glycerate to serine, or glycolate to glycine and serine in a sequence of reactions which involve chloroplasts, peroxisomes, mitochondria and cytosol (Tolber. 1971). The glycolate pathway also generates C1 compounds and serves as the generator of precursors for nucleic acid biosynthesis. Photorespiration- In green leaves, there are peroxisomes that carry out a process called photorespiration which is a light - stimulated production of CO2 that is 31 different from the generation of CO2 by mitochondria in the dark. In photorespiration, glycolic acid (glycolate), a two-carbon product of photosynthesis is released from chloroplasts and oxidized into and H2O2 by a peroxisomal enzyme called glycolic acid oxidase. Later on, glyoxylate is oxidized into CO2 and formate: CH2OH.COOH + O2 CHO - COOH + H2O2 CHO - COOH + H2O2 HCOOH + CO2 + H2O Photorepiration is so called because light induces the synthesis of glycolic acid in chloroplasts. This process involves intervention of two organelles : cloroplasts and peroxisomes. Lastly, photorespiration is driven by atmospheric conditions in which the O2 tension is high and CO2 tension low. Apparently O2 competes with CO2 for the enzyme ribulose diphosphate carboxylase which normally is the key enzyme in CO2 fixation during photosynthesis. When O2 is used by the enzyme, an unstable intermediate is formed which breaks down into 3-phosphoglycerate and phosphoglycolate. The latter tends to increase the glycolate concentration by removal of its phosphate group and, therefore, more glycolate is available for additional oxidation and CO2 release . Photorespiration is a wasteful process for the plant cell, since, it significantly reduces the efficiency of the process of photosynthesis (i.e., it returns a portion of fixed CO2 to the atmosphere). It is a particular problem in C3 plants that are more readily affected by low CO2 tensions ; C4 plants are much more efficient in this regard. Biogenesis of Peroxisomes At one time it was thought that the membrane 'shell' of the peroxisomes is formed by building of the endoplasmic reticulum (ER), while the 'content' or matrix is imported from the cytosol (cytoplasmic matrix). However, there is now evidence suggesting that new peroxisomes always arise from pre-existing ones, being formed by growth and fission of old organelles similar to mitochondria and chloroplasts. Thus, peroxisomes are a collection of organelles with a constant membrane and a variable enzymatic content. All of their proteins (both structural and enzymatic) are encoded by nuclear genes and are synthesized in the cytosol (cytoplasmic matrix) (i.e., on the free ribosomes). The proteins present in either lumen or membrane of the peroxisome are taken up post-translationally from the cytosol (cytoplasmic matrix) as the haeme-free monomer; the monomers are imported into the lumen of peroxisomes, where they assemble into tetramers in the presence of haeme./ Catalase and many peroxisomal proteins are found to have a signal 32 sequence (comprising of three amino acids) which is located near their carboxyl ends and directs them to peroxisome (Gould, Keller and Subramani,1988). Peroxisomes contain receptors exposed on their cytosolic surface to recognize the signal on the imported proteins. All of the membrane proteins of the peroxisomes. Including signal receptor proteins, are imported directly from the cytosol (cytoplasmic matrix). The lipids required to make new peroxisomal membrane are also imported from the cytosol (cytoplasmic matrix), possibly being carried by phospholipids transfer proteins from sites of their synthesis in the DR membranes (Affe and Kennedy, 1983). Glyoxysomes Glyoxysomes are found to occur in the cells of yeast, Neurospora, and oil rich seeds of many higher plants. They resemble with peroxisomes in morphological details, except that, their crystalloid core consists of dense rods of 6.0 m diameter. They have enzymes for fatty acid metabolism and gluconeogenesis, i.e. conversion of stored lipid molecules of spherosomes of germinating seeds into the molecules of carbohydrates. Functions Glyoxysomes perform following biochemical activities of plants cells : (1) Fatty acid metabolism. During germination of oily seeds, the stored lipid molecules of spherosomes are hydrolysed by the enzyme lipase (glycerol ester hydrolase) to glycerol and fatty acids. The phospholipids molecules are hydrolysed by the enzyme phospholipase. The long chain fatty acids which are released by the hydrolysis are then broken down by the successive removal of two carbon or C2 fragments in the process of -oxidation. During -oxidation process, the fatty acid is first activated by enzyme fatty acid thiokinase to fatty acyl-CoA which is oxidized by a FAD-linked enzyme fatty acyl-CoA dehydrogenase into-2-enoyl-CoA. Trans-2-enoyl-CoA is hydrated by an enzyme enoyl hydratase or crotonase to produce the L-3-hydroxyacyl-CoA, which is oxidized a NAD Linked L-3-hydratase or crotonase to produce the L-3-hydroxyacylCoA, which is oxidized by a NAD linked L-3-hydroxyacyl-CoA dehydrogenase to produce 3-Ketoacly-CoA. The 3-keto acyl-CoA looses a two carbon fragment under the action of the enzyme thiolase to generate an acetyl-CoA and a new fatty acylCoA with two less carbon atoms thatn the original. This new fatty acyl-CoA is then 33 recycled thought the same series of reactions until the final two molecules of acetylCoA are produced. In plant seeds -oxidation occurs in glyoxysomes (Cooper and Beevers, 1969). But in other plant cells -oxidation occurs in glyxysomes and mitochondria. The glyoxysomal -oxidation requires oxygen for oxidation of reduced flavorprotien produced as a result of the fatty-acyl-CoA dehydrogenase activity. In animal cells oxidation occurs in mitochondria. In plant cells, the acetyl-CoA, the product of -oxidation chain is not oxidized by Krebs cycle, because it remains spatially separated from the enzymes of Krebs cycle, instead of it, acetyl-CoA undergoes the glyoxylate cycle to be converted into succinate. (2) Glyoxylate cycle. The glyoxylate pathway occurs in glyoxysomes and it involve some of the reactions of the Krebs cycle in which citrate is formed from oxaloacetate and acetyl-CoA under the action of citrate synthetase enzymes. The citrate is subsequently converted into isocitrate by aconitase enzyme. The cycle then involves the enzymatic conversion of isocitrate to glyoxylate and succinate by isocitratase enzyme : Isocitratase Isocitrate Glyoxylate + Succinate The glyoxylate and another mole of acetyl-CoA form a mole of malate by malate synthesis ; Malate synthetase Acetyl CoA+Glyoxylate Malate This malate is converted to oxaloacetate by malate dehydrogenase for the cycle to be completed. Thus, overall, the glyoxylate pathway involves : 2 Acetyl-CoA+NAD+ Succintiate + NADH+ H+ Succinate is the end product of the glyoxysomal metabolism of fatty acid and is not further metabolized within this organelle. The synthesis of hexose or gluconeogenesis involves the conversion of succinate to oxaloacetate, which presumably takes place in the mitochondria, since the glyoxsomes do not contain the enzymes fumarase and succinic dehydrogenase. Two molecules of oxaloacetate are formed from four molecules of acetyl-CoA 34 without carbon loss. This oxaloacetate is converted to phosphoenol pyruvate in the phosphoenol pyruvate caboxykinase reaction with the loss of two molecules of CO2 : 2 Oxaloacete + 2ATP 5.3 2 Phosphoenol pyruvate + 2CO2 + 2ADP Golgi Apparatus For the performance of certain important cellular functions such as biosynthesis of polysaccharides, packaging (compartmentalizing) of cellular synthetic products (proteins), production of exocytotic (secretory) vesicles and differentiation of cellular membranes, there occurs a complex organelle called Golgi complex or Golgi apparatus in the in the cytoplasm of animal and plant cells. The Golgi apparatus, like the endoplasmic reticulum, is a canalicular system with sacs, but unlike the endoplasmic reticulum it has parallely arranged, flattened, membrane-bounded vesicles which lack ribosomes and stainable by osmium tetraoxide and silver salts. History An Italian neurologist (i.e., physician) Camillo Golgi in 1873 discovered and developed the silver chromate method (termed la reazione nera) for studying histological details of nerve cells. of cerebral cortex of brain) of barn owl contained and internal reticular network which stains black with the silver stain. He called this structure apparato reticolare interno (internal reticular apparatus). By reporting the existence of such an organelle inside cell, he inadvertently raised a storm of controversy in the scientific world, which is commonly known as the Golgi controversy. Occurence The Golgi apparatus occurs in all cells except the prokaryotic cells (viz., mycoplasmas, bacteria and blue green algae) and eukaryotic cells of certain fungi, sperm cells of bryophytes and pteridiophytes, cells of mature sieve tubes of plants and mature sperm and red blood cells of animals. Their number per plant cell can vary from several hundred as in tissues of corn root and algal rhizoids (i.e., more than 25,000 in algal rhizoids, Sievers, 1965), to a single organelle in some algae. (Certain algal cells such as Pinularia and Microsterias, contain largest and most complicated Golgi apparatuses. In higher plants, Golgi apparatuses are particularly common in secretory cells and in young rapidly growing cells. In animal cells, there usually occurs a single Golgi apparatus, however, its number may vary from animal to animal and from cell to cell. Thus Paramoeba 35 species has two Golgi apparatus and nerve cells, liver cells and chordate oocytes have multiple Golgi apparatuses, there being about 50 of them in the liver cells. Distribution In the cells of higher plants, the Golgi bodies or dictyosomes are usually found scattered throughout the cytoplasm and their distribution does not seem to be ordered or localized in any particular manner (Hall et al., 1974). However, in animal cells the Golgi apparatus is a localized organelle. For example, in the cells of ectodermal or endodermal origin, the Golgi apparatus remains polar and occurs in between the nucleus and the periphery (e.g., thyroid cells, exocrine pancreatic cells and mucus-producing goblet cells of intestinal epithelium) and in the nerve cells it occupies a circum-nuclear position. Morphology The Golgi apparatus is morphologically very similar in both plant and animal cells. However, it is extremely pleomorphic: in some cell types it appears compact and limited, in others spread out and reticular (net-like). Its shape and form may very depending on cell type. Typically, however, Golgi apparatus appears as a complex array of interconnecting tubules, vesicles and cisternae. There has been much debate concerning the terminology of the Golgi's parts. The classification given by D.J. Morre (1977) is most widely used. In this scheme, the simplest unit of the Golgi apparatus is the cisterna. This is a membrane bound space in which various materials and secretions may accumulate. Numerous cisternae are associated with each other and appear in a stack like (lamellar) aggregation. A group these cisternae is called the dictyosome, and a group of dictyosomes makes up the cells Golgi apparatus. All dictyosomes of a cell have a common function (see Berns, 1983). 1. Flattened Sac or Cisternae Cisternae (aboute 1m in diameter) are central, flattened, plate-like or saucer-like closed compartments which are help in parallel bundles or stacks on above the other. In each stack, cisternae, are separated by a space of 20 to 30 nm which may contain rod-like elements or fibres. Each stack of cisternae forms a dictyosome which may contain 5 to 6 Golgi cisternae in animal cells or 20 or more cisternae in plant cells. Each cisterna is bounded by a smooth unit membrane (7.5 nm thick), having a lumen varying in width from about 500 to 1000 nm (see Sheeler and Bianchi, 1987). The margins of each cisterna are gently curved so that the entire dictyosome of Golgi apparatus takes on a bow like appearance. The cisternae at the 36 convex end of the dictyosome comprise proximal forming or cis-face and the cisternae at the concave end of the dictyosome comprise the distal, maturing or trans-face. The forming or cis-face of Golgi is located next to either the nucleus or a specialized portion of rough ER that lacks bound ribosomes and is called ''transitional'' ER. Trans face of Golgi is located near the plasma membrane. This polarization is called cis-trans axis of the Golgi apparatus. 2. Tubules A complex array of associated vesicles and anastomosing tubules (30 to 50nm diameter) surround the dictyosome and radiate from it. In fact, the peripheral area of dictyosome is fenestrated (lace-like) in structure. 3. Vesicles The vesicles (60 nm in diameter) are of three types : (i) Transitional vesicles are small membrane limited vesicles which are through to form as blebs from the transitional ER to migrate and converge to cis face of Golgi, where they coalesce to form new cisternae. (ii) Secretory vesicles are varied-sized membrane-limited vesicles which discharge from margins of cisternae of Golgi. They, often, occur between the maturing face of Golgi and the plasma membrane. (iii) Clathrin-coated vesicles are spherical protuberances, about 50m in diameter and with a rough surface. They are found at the periphery of the organelle, usually at the ends of single tubules, and are morphologically quite distinct from the secretory vesicles. The clathrincoated vesicles are known to play a role in intra-cellular traffic or membranes and of secretory products. i.e., between ER and Golgi, as well as between GELR region and the endosomal and lysosomal compartments. Isolation andChemical Composition Initially, Golgi apparatus was isolated only from cells of the epididymis, however in recent years, it has been isolated from number of plant and animal cells. The isolation of Golgi apparatus brough about mainly by gentle homogenization following by differential and gradient homogenization Gentle homogenization is preferred to preserve the stacks of cisternae, Due to its low density, Golgi apparatuses tend to form a distinct band in gradient centrifugation. The isolated Golgi apparatus is 37 apparatus tend to form a distinct band in gradient centrifugation. The isolated Golgi apparatus washed with distilled water for purifying it, though, its secretory components are lost (see Thorpe, 1984). Chemically, Golgi apparatus of rat liver contains about 60 per cent lipid material. The Golgi apparatus of animal cells contains phospholipids in the form of phosphatidyl choline, whereas, that of plant cells contains phosphatidic acid and phosphatidyl glycerol. The Golgi apparatus also contains a variety of enzyme (Table 7-1), some of which have been used as cytochemical markers. Cytochemical Properties of Golgi Apparatus Different parts of Golgi apparatus have been histochemically identified by specific staining properties (Thorpe, 1984 Alberts et al., 1989) : 1. Osmium tetraxide (OsO4) selectively impregnates the outer face (cis-face) of the Golgi apparatus. This stain adheres well to lipids, especially phospholipids and unsaturated fats. 2. Phosphotungstic acid (H3PO4.12WO3.24H2O). selectively stain the maturing or trans face of Golgi stack. This stain is an anionic stain having special affinity for polysaccharides and proteins. 3. Glycosyl tranferease and thiamine pyrophosphatase can be localized cytochemically in the trans cisternae of Golgi apparatus. Transferase enzyme are found to be located in the membrane of Golgi, not in the lumen of cisternae (Thorpe, 1987). 4. Acide phosphatase enzyme is cytochemically marked in the GERL region. Origin Origin of Golgi apparatus involves the formation of new cisternae and there is great variation in shape, number and size of cisternae in each stack (dictyosome). The process of formation of new cisternae may be performed by any of the following methods ; 1. Individual stacks of cisternae may arise from the pre-existing stacks by division or fragmentation. 2. The alternative method of origin of Golgi is based on denovo formation. In fact various cytological and biochemical envidences have established that the membranes of the Golgi apparatus are originated from the membranes of the smooth ER which in turn have originated from the rough ER. The proximal Golgi saccules are formed by fusion of ER-derived vesicles, while distal saccules "give their all" to vesicle formation and disappear. Thus, Golgi saccules are constantly and rapidly renewed. 38 The cells of dormant seeds of higher plants generally lack Golgi apparatuses but they do display zone of exclusion having aggregation of small transition vesicles. Photomicrographs of cells in early stages of germination suggest progressive development of Golgi bodies in these zones of exclusion ; and the development of Golgi apparatuses coincides with the disappearance of the aggregation of vesicles (see Sheeler and Bianchi, 1987). Function Golgi vesicles are often, referred to as the ''traffic police" of the cell (Dernell et al., 1986. They play a key role in sorting many of cell's proteins and membrane constituents, and in directing them to their proper destinations. To perform this function, the Golgi vesicles contain different sets of enzymes in different types of vesicles-cis, middle and trans cisternae- that react with and modify secretory proteins passing through the Golgi lumen or membrane proteins and glycoprotein's in the Golgi membranes as they are on route to their final destinations. For example a Golgi enzyme may add a "signal" or "tag" such as a carbohydrate or phosphate residues to certain proteins to direct them to their proper sites in the cell. Or, a proteolytic Golgi enzyme may cut a secretory or membrane protein into two or more specific segments (E.g., molecular processing involved in the formation of pancreatic hormone insulin: preproinsulinproinsulininsulin). Recently, in the function of Golgi apparatus, sub compartmentalization with a division of labour has been proposed between the cis region (in which proteins of RER are sorted and some of them are returned back possibly by coated vesicles), and the trans region in which the most refined proteins are further separated for their delivery to the various cell compartments (e.g., plasma membrane, secretory granules and lysosomes). 39 Thus, Golgi apparatus is a centre of reception, finishing, packaging, and dispatch for a variety of materials in animal and plant cells : 1. Golgi Functions in Plants In plants, Golgi apparatus is mainly involved in the secretion of mainly involved in the secretion of materials of primary and secondary cell walls (e.g., formation and export of glycoprotein, lipids, pectins and monomers for hemicellulose, cellulose, lignin, etc). During cytokinesis of mitosis or meiosis, the vesicles originating from the periphery of Golgi apparatus, coalesce in the phragmoplast area to form a semisolid layer, called cell plate. The unit membrane of Golgi vesicles fuses during cell plate formation and becomes part of plasma membrane of daughter cells . 5.4 Lysosomes The lysosomes (Gr. lyso=digestive + soma = bodies) are tiny membrane-bound vesicles involved in intracellular digestion. They contain a variety of hydrolytic enzymes that remain active under acidic conditions. The lysosomal lumen is maintained at an acidic pH (around 5) by an ATP-driven proton pump in the membrane. Thus, these remarkable organelles are primarily meant for the digestion of a variety of biological materials and secondarily cause aging and death of animal cells and also a variety of human diseases such as cancer, gout Pompe's silicosis and I-cell disease. History During early electron microscopic studies, rounded dense bodies were observed in rat liver cells. These bodies were initially described as "perinulclear 40 dense bodies", C. de Duve, in 1955, renamed these organelles as "lysosomes" to indicate that the internal digestive enzymes only became apparent when the membrane of these organelles was lysed (See Reid and Leech, 1980). However, the term lysosome means lytic body having digestive enzymes capable of lysis (viz., dissolution of a cell or tissue ; (De Robertis and De Robertis, Jr., 1987). Lysosomes were investigated according to following two schools (1) C, de Duve and his coworkers (1963, 1964) worked in Belgium and their approach was biochemical one . (2) Alex Novikoff and his research group (1962, 1964) worked in United States and their approach was morphological and cytochemical. For the discovery of lysosomes and a brilliant series of experiments on them, de Duve shared the 1974 Nobel Prize for physicality with Palade andClaude, both were pioneer cells biologists. Occurence The lysosomes occur in most animal and few plant cells. They are absent in bacteria and mature mammalian ertythrocytes. Few lysosomes occur in muscle cells or in cells of the pancreas. Leucocytes, especially granulocytes are a particularly rich source of lysosomes. Their lysosomes are so large sized that they can be observed under the light microscope. Lysosomes are also numerous in epithelial cells of absorptive, secretory and excretory organs (e.g., intestine, liver, kidney, etc.) They occur in abundance in the epithelial cells of lungs and uterus. Lastly phagocytic cells and cells of reticuloendothelial system (e.g., bone marrow, spleen and liver) are also rich in lysosomes. Structure The lysosomes are round vacuolar structure which remain filled with dense material and are bounded by single unit membrane. Their shape and density vary greatly. Lysosmes are 0.2 to 0.5m in size. Since, size and shape of lysosomes vary from cell to cell and time to time (i.e. they are polymorphic), their identification becomes difficult. However, on the basis of the following three criteria, a cellular entity can be identified as a lysosome : (1) It should be bound by a limiting membrane (2) It should contain two or more acid hydrolases ; and (3) It should demonstrate the property of enzyme latency when treated in away that adversely affects organelle's membrane structure. 41 Isolation and Chemical Composition Lysosomes are very delicate and fragile organelles. Lysosomal fraction have been isolated by sucrose-density centrifugation (or Isopycnic centrifugation) after mild methods of homogenization. Since the original de Duve's isolated lysosomal fractions were having contaminations of mitochondria, microsomes and microbodies, so, 1960's it was investigated that rats injected with dextran or Triton WR-1339, incorporated these compounds into their lysosomes, thereby altering their density and making their cleaner separation possible by differential centrifugation and density gradients (see Reid and Leech, 1980). Lysosomes tend to accumulate certain dyes (vital stains such as Neutral red, Niagara, Evans blue) and drugs such as anti-malarial drug chloroquine. Such 'loaded' lysosomes can be demonstreated by fluorescence microscopy. The location of the lysosomes in the cell can also be pinpointed by various histochemical or cytochemical methods. For example, lysosomes demonstrate the property of metachromasia with toluidine blue and give a positive acid Schiff reaction (see Chapter 2). Metachromasia is the property exhibited by certain pure dyestuffs, chiefly basic stains, of colouring certain tissue elements in a different colour. Certain lysosomal enzymes are good histochemical markers. For example, acid phosphatase is the principal enzyme which is used as a marker for the lysosomes by the use of Gomori staining technique (Gomori, 1952). Specific stains are also used for other lysosomal enzymes such as B-glucuronidase, aryl sulphatatase, N-acetylB-glucosaminidase and 5-bromo-4-chloroindolacetate esterase. Lysosomal Enzymes According to a recent estimate, a lysosome may contain up to 40 types of hydrolytic enzymes (see Alberts et al., 1989). they include proteases (e.g., cathespsin for protein digestion), nucleases, glycosidases (for digestion of polysaccharides and glycosides ), lipase, phospholipases, phosphatases and sulphatases (table 8-2). All lysosomal enzymes are acid hydrolases, optimally active at the pH5 maintained within lysosomes. The lysosomal enzymes latent and out of the cytoplasmic matrix or cytosol (whose pH is about ~ 7.2), but the acid dependency of lysosmal enzymes protects the contents of the cytosol (cytoplasmic matrix) against any damage even if leakage of lysosomal enzymes should occur. The so-called latency of the lysosomal enzymes is due to the presence of the membrane which is resistant to the enzymes that it encloses. Most probably this is to 42 the face that most lysosomal hydrolases are membrane-bound, which may prevalent the active centre of enzymes to gain access to susceptible groups in the membrane ( Reid and Leech, 1980). Kind of Lysosomes Lysosomes are extremely dynamic organelles, exhibiting polymorphism in their morphology. Following four types of lysosomes have been recognized in different types of cells or at different time in the same cell. Of these, only the first is the primary lysosome, the other three have been grouped together as secondary lysosomes. 1. Primary Lysosomes These are also called storage granules, protolysosomes or virgin lysosomes. Primary lysosomes are newly formed organelles bounded by a single membrane and typically having a diameter of 100nm. They contain the degradative enzymes which have not participated in any digestive process. Each primary lysosome contains one type of enzyme or another and it is only in the secondary lysosome that the full complement of acid hydrolases is present. 2. Heterophagosomes They are also called heterophagic vacuoles, heterolysosomes or phagolysosomes. Heterophagosomes are formed by the fusion of primary lysosomes with cytoplasmic vacuoles containing extracellular particles into the cell by any of a variety of endocytic processes (e.g., pinocytosis, phagocytosis or receptor mediated endocytosis. The digestion of engulfed substances takes place by the enzymatic activities of the hydrolytic enzymes of the secondary lysosomes. The digested material has low molecular weight and readily passes through the membrane of the lysosomes to become the part of the matrix. 3. Autophagosomes They are also called autophagic vacuole, cytolysosomes or autolysosomes. Primary lysosomes are able to digest intracellular structures including mitochondria, ribosomes, peroxisomes and glycogen granules. Such autodigestion (called autophagy) of cellular organelles is a normal event during cell growth and repair and is especially prevalent in differentiating and de-differentiating tissues (e.g., cells undergoing programmed death during 43 meta-morphosis or regeneration) and tissue under stress. Autophagy takes several forms. In some cases the lysosome appears to flow around the cell structure and fuse, enclosing it in a double membrane sac, the lysosomal enzymes being initially confined between the membrane. The inner membrane then breaks down and the enzymes are able to penetrate to the enclosed organelle. In other cases, the organelle to be digested is first encased by smooth ER, forming a vesicle that fuses with a primary lysosome. Lysosomes also regularly engulf bits of cytosol (cytoplasmic matrix) which is degraded by a process, called microautophagy. As digestion proceeds, it becomes increasingly difficult to identify the nature of the original secondary lysosome (i.e., heterophagosome or autophagosome) and the more general term digestive vacuole is used to describe the organelle at this stage. 4. Residual Bodies They are also called telolysosomes or dense bodies. Residual bodies are forced if the digestion inside the food vacuole is incomplete. Incomplete digestion may be due to absence of some lysosomal enzymes. The undigested food is present in the digestive vacuole as the residues and may take the form of whorls of membranes. grains, amorphouse masses, ferritinlike or myelin figures. Residual bodies are large, irregular in shape and are usually quite electrondense. In some cells, such as Amoeba and other potozoa, these residual bodies are eliminated defecation. In other cells, residual bodies may remain for a long time and may load the cells to result in their aging. For example, pigment inclusions (age pigment or lipofuscin granules) found in nerve cells (also in liver cells, heart cells and muscle cells) of old animals may be due to the accumulation of residual bodies. Origin The biogenesis (origin) of the lysosomes requires the synthesis of specialized lysosomal hydrolases and membrane proteins. Both classes of proteins are synthesized in the ER and transported through the Golgi apparatus, then transported from the trans Golgi network to an intermediate compartment (an endolysosome) by means of transport vesicles (which are coated by clathrin protein). The lysosmal 44 enzymes are glycol proteins, containing N-linked oligosaccharides that are processed in a unique way in the cis Golgi so that their mannose residues are phophorylated. These mannose 6-phosphate (M6P) groups are recognized by M6P groups are recognized by M6P-receptors (which are trans membrane proteins) in the trans Golgi network that segregates the hydrolases and helps to package them into budding clathrin-coated vesicles which quickly lose their coats. These transport vesicles containing the M6P-receptors act as shuttles that move the receptors back and forth between the Golgi network and endolysosomes. They low pH in the endolysosome dissociates the lysosomal hydrolases from this receptor, making the transport of the hydrolases unidirectional. Function of Lysosomes The important functions of lysosomes are as follow : 1. Digestion of large extracellular particles. The lysosomes digests the food contents of the phoagosomes or pinosomes. 2. Digestion of intracellular substance. During the starvation, the lysosomes digest the stored food contents viz proteins, lipids and carbohydrates (glycogen) of the cytoplasm and supply to the cell necessary amount of energy. 3. Autolysis. In certain pathological conditions the lysosomes start to digest the various organelles of the cells and this process is known as autolysis or cellular autophagy. When a cell dies, the lysosome membrane ruptures and enzymes are liberated. These enzymes digest the dead cells. In the process of metamorphosis of amphibians and / tunicates many embryonic tissues, e.g., gills, fins, tail, etc., are digested by the lysosomes and utilized by the other cells. 4. Extracellular Digestion. The lysosomes of certain cells such as sperms discharge their enzymes outside the cell during the process of fertilization. The lysosomal enzymes digest the limiting membrane of the ovum and form penetration path in ovum for the sperms. Acid hydrolases are released from osteoclasts and break down bone for the reabsorption ; these cells also secrete lactic acid which makes the local pH enough for optimal enzyme activity. Likewise, preceding ossification (bone formation), fibroblasts release cathepsin D enzyme to break down the connective tissue. 45 Lysosomes in plants Plants contain several hydrolases, but they are not always as neatly compartmentalized as they are in animal cells. Many of these hydrolases are found bound to and functioning within the vicinity of the cells wall and are not necessarily contained in membrane bound vacuoles at these sites. Many types of vacuoles and storage granules of plants are found to contain certain digestive enzymes and these granules are considered as lysosomes of plant cell (Gahan, 1972). According to Matile (1969) the plant lysosomes can be defined as membrane bound cell compartments containing hydrolytic digestive enzymes. Matile (1975) has divided vacuoles of plants into following three types : 1. Vacuoles The vacuole of a mature plant cell is formed from the enlargement and fusion of smaller vacuoles present in meristematic cells; these provacuolse, which are believed to be derived from the ER and possibly the Golgi and contain acid hydrolases. These lysosomal enzymes are associated with the tonoplast of large vacuole of differentiating cells. Sometimes, mitochondria and plastids are observed inside the vacuole suggesting autophagy in plants (Swanson and Webster,1989). 2. Spherosomes 46 The spherosomes are membrane bounded, spherical particles of 0.5 to 2.5 m diameter, occurring in most plant cells. They have a fine granular structure internally which is rich in lipids and proteins. They originate from the endoplasmic reticulum (ER). Oil accumulates at the end of a strand of ER and a small vesicle is then cut off by contribution to form particles, called prospherosomes. The prospherosomes grow in size to form spherosoms. Basiocally, the spherosomes are involved in lipid synthesis and storage. But, the spherosomes of maize root tips (Matile, 1968) and spherosome of tobacco endosperm tissue (Spichiger, 1969) have been found rich in hydrolytic digestive enzymes and so have been considered as lysosomes. Like lysosomes they are not only responsible for the accumulation and mobilization of reserve lipids, but also for the digestion of other cytoplasmic components incorporated by phagocytosis. 3. Aleurone Grain The aleurone grains or protein bodies are spherical membrane-bounded storage particle occurring in the cells of endosperm and cytoledons of seeds. They are formed during the later stages of seed ripening and disappear in the early stages of germination. They store protein (e.g., globulins) and phosphate in the form of phytin. Matile (1968) has demonstrated that aleurone grains from pea seed contain a wide range of hydrolytic enzymes including protease and phosphatase which are required for the mobilization of stored protein and phosphate, although the presence of other enzymes such as amylase and RNAase suggest that other cell constituents may also be digested. Thus like spherosomes, aleurone grains store reserve materials, mobilize them during germination and in addition form a compartment for the digestion of other cell components (Hall et al., 1974). The aleurone grains are derived from the strands of the endoplasmic reticulum. During germinating of barley seed, the activity of hydrolases is found to be controlled by hormones such as gibberellic acid. Gibberellic acid, a plant growth hormone, is released by the embryo to the aleurone layer where, in turn, the hyrolases are released to the endosperm. This hormone operates by derepressing appropriate genes in the aleurone cells, which then begin to crank out new hydrolytic proteins (see Thorpe, 1984). 5.5 Endoplasmic Reticulum 47 The cytoplasmic matrix is traversed by a complex network of inter-connecting membrane bound vacuoles or cavities. often remain concentrated in the endoplasmic portion of the cytoplasm ; therefore, known as endoplasmic reticulum, a name derived from the fact that in the light microscope it looks like a "net in the cytoplasm." (Eighteenth-century European ladies carried purses of netting called reticules). The name "endoplasmic reticulum" was coined in 1953 by Porter, who in 1945 had observed it in electron micrographs of liver cells. Fawcet and Ito (1958), Thiery (1958) and Rose and Pomerat (1960) have made various important contributions to the endoplasmic reticulum. The occurrence of the endoplasmic reticulum varies from cell to cell. The erythrocytes (RBC), egg and embryonic cells lack in endoplasmic reticulum. The spermatocytes have poorly developed endoplasmic reticulum. The adipose tissues, brown fat cells and adrenocortical cells, interstitial cells of testes and cells of corpus luteum of ovaries, sebaceous cells and retinal pigment cells contain only smooth endoplasmic reticulum (SER). The cells of those organs which are actively engaged in the synthesis of proteins such as chinar cells of pancreas, plasma cells, goblet cells and cells of some endocrine glands are found to contain rough endoplasmic reticulum (RER) which is highly developed. The presence of both SER and RER in the hepatocytes (liver cells) is reflective of the variety of the roles played by the liver in metabolism. Morphology Morphologically, the endoplasmic reticulum may occur in the following three forms : 1. Lamellar form or cisternae (A closed, fluid-filled sac, vesicle or cavity is called cisternae) 2. vesicular form or vesicle and 3. tubular form or tubules. 1. Cisternae. The cisternae are long, flattened, sac-like, unbranched tubules having the diameter of 40 to 50 m. They remain arranged parallely in bundles or stakes. PER usually exists as cisternae which occur in those cells of pancreas, notochord and brain. 2. Vesicles. The vesicles are oval, membrane bound vacuolar structures having the diameter of 25 to 500m. They often remain isolated in the cytoplasm and occur in most cells but especially abundant in the SER. 48 3. Tubules. The tubules are branched structures forming the reticular system along with the cisternae and vesicles. They usually have the diameter from 50 to 190m and occur almost in all the cells. Tubular form of ER is often found in SER and is dynamic in nature, i.e., it is associated with membrane movements, fission and fusion between membranes of cytocavity network (see Thorpe, 1984). Ultra structure The cavities of cisternae, vesicles and tubules of the endoplasmic reticulum are bounded by a thin membrane of 50 to 60 A 0 thickness. The membrane of endoplasmic reticulum is fluid-mosaic like the unit membrane of the plasma membrane, nucleus, Golgi apparatus, etc. The membrane, thus, is composed of a bimolecular layer of phospholipids in which 'float' proteins of various sorts. The membrane of endoplasmic reticulum remains continuous with the membranes of plasma membrane, nuclear membrane and Golgi apparatus. The cavity of the endoplasmic reticulum is well developed and acts as a passage for the secretory products. Palade (1956) has observed secretory granules in the cavity of endoplasmic reticulum. Sometimes, the cavity of RER is very narrow with two membranes closely apposed and is much distended in certain cells which are actively engaged in protein sysnthesis (e.g., acinar cells, plasma cells and goblet cells). Weibel et al. , 1969, have calculated that the total surface of ER contained in 1ml of liver tissue is about 11 square metres, two-third of which is or rough types (i.e., RER). Types of Endoplasmic reticulum Two types of endoplasmic reticulum have been observed in same or different types of cells which are as follows : 1. Agranular or Smooth Endoplasmic Reticulum This type of endoplasmic reticulum possesses smooth walls because the ribosomes are not attached with its membranes. The smooth type of endoplasmic reticulum occurs mostly in those cells, which are involved in the metabolism of lipids (including steroids) and glycogen. The smooth endoplasmic reticulum is general found in adipose cells, interstitial cells, glycogen storing cells of the liver, conduction fibres of heart, spermatocytes and leucocytes. The muscle cells are also rich in smooth type of endoplasmic reticulum and here it is known as sarcoplasmic 49 reticulum. In the pigmented retinal cells it exists in the form of tightly packed vesicles and tubes known as myeloid bodies. Glycosomes. Although the SER forms a continuous system with RER, it has different morphology. For example, in liver cells it consists of a tubular network that pervades major portion of the cytoplasmic matrix. These fine tubules are present in regions rich in glycogen and can be observed as dense particles, called glycosomes, in the matrix. Glycosomes measure 50 to 200 mm in diameter and contain glycogen along with enzymes involved in the synthesis of glycogen (Rybicka, 1981). Many glycosomes attached to the membranes of SER have been observed by electron microscopy in the liver and conduction fibre of heart. 2. Granular of Rough Endoplasmic Reticulum The granular or rough type of endoplasmic reticulum possesses rough walls because the ribosomes remain attached with its membranes. Ribosomes play a vital role in the process of protein synthesis. The granular or rough type of endoplasmic reticulum is found abundantly in those cells which are active in protein sysnthesis such as pancreatic cells, plasma cells, goblet cells, and liver cells. The granular type of endoplasmic reticulum takes basiophilic stain due to its RNA content of ribosomes. The region of the matrix containing granular type of endoplasmic reticulum takes basiophilic stain and is names as ergastoplasm, basiophilic bodies, chromophilic substances or Nissl bodies by early cytologists. In RER, ribosomes are often present as polysomes held together by mRNA and are arranged in typical, "rosettes" of spirals. RER contains two transmembrane glycoproteins (called ribophorins I and II of 65,000 and 64,000 dalton MW, respectively), to which are attached the ribosomes by their 60S subunits. Isolation and chemical composition The membranes of the endoplasmic reticulum can be isolated by subjecting homogenized tissues to differential centrifugation. Electron microscopy of such ER preparations reveals that the membranes disrupt to form closed vesicles (~100 nm diameter) of either a rough or a smooth form. These membranous entities were coined the term "microsomes" by Claude in 1940, and the relationship between microsomes and the elements of endoplasmic reticulum in the intact cell was established by Palade and Siekevitz 1956. Microsomes derived from rough ER are studded with and are called rough or granular microsomes. The ribosomes are always found on the outside surface, the interior beings biochemical equivalent to the luminal space of the ER. Homogenate 50 also contains smooth or agranular micro-somes which lack attached ribosomes. They may be derived in part from smooth portion of the ER and in part from fragments of plasma membrane, Golgi apparatus, endosomes and mitochondria. Thus, while rough microsomes can be equated with rough portions of ER, the origin of smooth microsomes cannot be so easily assigned. However, since the hepatocytes of liver contain exceedingly large quantities of smooth ER, therefore, most of the smooth microsomes in liver homogenates are derived from smooth ER (see Alberts et al., 1989). Enzymes of the ER membranes The membranes of the endoplasmic reticulum are found to contain many kinds of enzymes which are needed for various important synthetic activities. Some of the most common enzymes are found to have different transverse distribution in the ER membranes (Table 6-1). The most important enzymes are the stearases, NADH-cytochrome C reductase, NADH diaphorase, glucose-6-phosphotase and Mg++ activated ATPase. Certain enzymes of the endoplasmic reticulum such as nucleotide diphosphate are involved in the biosynthesis of phospholipids, ascorbic acid, glucuronide, steroids and hexose metabolism. The enzymes of the endoplasmic reticulum perform the following important functions : 1. Synthesis of glycerides, e.g., triglycerides, phospholipids, glycolipids and plasmalogens. 2. Metabolism of plasmalogens. 3. Sythesis of fatty acids. 4. Biosynthesis of the steroids, e.g., cholesterol biosynthesis, steroid hydrogenation of unsaturated bonds. 5. NADPH2+O2- requiring steroid transformations : Aromatization and hydroxylation. 6. NADPH2+O2-requireing steroid transformations : Aromatization hydroxylation's side-chain oxidation, thio-ether oxidations, desulphuration. 7. L-ascorbic acid metabolism. 8. UDP-glucose dephosphorylation. 9. Ary1- and steroid sulphatase. Origin of Endoplasmic reticulum 51 The exact process of the origin of endoplasmic reticulum is still unknown. But because membranes of ER resemble with the nuclear membrane and plasma membrane and also at the telophase stage the ER membranes are found the nuclear envelope. Therefore, it is normally assumed that the ER has originated by evagination of the nuclear membranes. Teikevitz and Palade (1960) have reported that the granular type of ER has originated first and later it synthesizes the agranular or smooth type of endoplasmic reticulum. The synthesis of membranes of ER is found to proceed in the following direction : RERSER. In face, membrane biogenesis is a multi-step process involving, first, the synthesis of a basic membrane of lipid and intrinsic proteins and thereafter the addition of other constituents such as enzymes, specific sugars, or lipids. The process by which a membrane is modified chemically and structurally is called membrane differentiation. The insertion of proteins into ER membranes occurs at the level of RER. Most of these proteins are formed on membrane-bound ribosomes. However, some of these are synthesized by free ribosomes in the cytosol (cytoplasmic matrix) and then are inserted into the membrane. For example, the enzyme NAD-cytochrome-b5-reductase is synthesized in the cytosol (cytoplasmic matrix) and then becomes incorporated in various parts of the endomembrane system (i.e., RER, SER and Golgi apparatus) and in the outer mitochondrial membrane (Borghese and Gaetani, 1980). Function of Endoplasmic reticulum The endoplasmic reticulum acts as secretory, storage, circulatory and nervous system for the cell. performs following important functions : A. Common Functions of Granular and Agranular Endoplasmic Reticulum 1. The endoplasmic reticulum provides and ultrastructural skeletal framework to the cell and gives mechanical support to the colloidal cytoplasmic matrix. 2. The exchange of molecules by the process of osmosis, diffusion and active transport occurs through the membranes of endoplasmic reticulum. Like plasma membrane, the ER membrane has permeases and carries. 52 3. The endoplasmic membranes contain many enzymes which perform various synthetic and metabolic activities. Further the endoplasmic reticulum provides increase surface for various enzymatic reactions. 4. The endoplasmic reticulum acts as an intracellular circulatory or transporting system. Various secretory products of granular endoplasmic reticulum are transported to various organelles as follows : Granular ERagranular ERGolgi membranelysosomes, transport vesicles or secretory granules. Membrane flow many also be an important mechanism for carrying particles, molecules and ions into and out of the cells. Export of RNA and nucleoproteins from nucleus to cytoplasm may also occur by this type of flow (see De Robertis and De Robertis, Jr., 1987). 5. The ER membranes are found to conduct intra-cellular impulses. For example, the sarcoplasmic reticulum transmits impulses from the surface membrane into the deep region of the muscle fibres. 6. The ER membranes form the new nuclear envelope after each nuclear division. 7. The sarcoplasmic reticulum plays a role in releasing calcium when the muscle is stimulated and actively transporting calcium back into the sarcoplasmic reticulum when the stimulation stops and the muscle must be relaxed. B. Functions of Smooth Endoplasmicreticulum Smooth ER performs the following functions of the cell : 1. Synthesis of lipids. SER perform synthesis of lipids (e.g., phospholipids, cholesterol, etc.) and lipoproteins. Studies with radioactive precursors have indicated that the newly synthesized phospholipids are rapidly transferred to other cellular membranes by the help of specific cytosolic enzymes, called phospholipids exchange proteins. 2. Glycogenolysis and blood glucose homeostasis. This process of glycogen synthesis (glycogenesis) occurs in the cytosol (in glycosomes). The enzyme UDPG-glycogen transferase, which is directly involved in the synthesis of glycogen by addition of uridine diphosphate glucose (UDPG) to primer glycogen is bound to the glycogen particles or glycosomes. 53 SER is found related to glycogenolysis or breakdown of glycogen. An enzyme, called glucose-6-phosphatase (a marker enzyme) exists as an integral protein of the membrane of SER (e.g., liver cell). Generally, this enzyme acts as a glycogenic phosphorhydrolase that catalyzes the release of free glucose molecule in the lumen of SER from its phosphorylated form in liver. Thus, this process operates to maintain homeostatic levels of glucose in the blood for the maintenance of functions of red blood cells and nerve tissues. 3. Sterol metabolism. The SER contains several key enzymes that catalyze the synthesis of cholesterol which is also a precursor substance for the biosynthesis of two types of compounds- the steroid hormones and bile acids : (i) Cholesterol biosynthesis. The cholesterol is synthesized from the acetate and its entire biosynthetic pathway involve about 20 steps, each step catalyzed by an enzyme. Out of these twenty enzymes, eleven enzymes are bounded to SER membranes, rest nine enzymes are the soluble enzymes located in the cytosol and mitochondria. Examples of SER-bound enzyme include HMG-Co A reductase and squalene synthetase (see Thorpe, 1984). (ii) Bile acid synthesis. The biosynthesis of the bile acids represents a very complex pattern of enzymes and products. Enzymes involved in the biosynthetic pathway of bile acids are hydroxylases, monooxygenases, dehydrogenases, isomerases and reductases. For example, by the help of the enzyme cholesterol 7-hydroxylase, the cholesterol is first converted into 7-hydroxyl cholesterol, which is then converted into bile acids by the help of hydroxylase enzymes. The latter reaction requires NADPH and molecular oxygen and depends on the enzymes of Electron transport chains of SER such as cytochrome P-450 and NADPH-cytochrome-c reductase . (iii) Steroid hormone biosynthesis. Steroid hormones are synthesized in the cells of various organs such as the cortex of adrenal gland, the ovaries, the testes and the placenta. For example, cholesterol is the precursor for both types of sex hormones-estrogen and testosterone54 made in the reproductive tissues, and the adrenocorticoids (e.g., corticosterone, aldosterone and cortisol) formed in the adrenal glands. Many enzymes (e.g., dehydrogenase,s isomerases and hydroxylases) are involved in the biosynthetic pathway of steroid hormones, some of which are located in SER membranes and some occur in the mitochondria 4. Detoxification. Protectively, the ER chemically modifies xenobiotics (toxic materials of both endogenous and exogenous origin), making them more hydrophilic, hence, more readily excreted. Among these materials are drugs, aspirin (acetyl-salicylic-acid), insecticides, anaesthetics, petroleum products, pollutant and carcinogens (i.e., inducers of cancer ; e.g., 3-4-benzophrene and 3-methyl cholantherene). The enzymes involved in the detoxification of aromatic hydrocarbonds are aryhydraoxylases. It is now know that benzophyrene (found in charcoalbroiled meat) is not carcinogenic, but under the action of aryl hydroxylase enzyme in the liver, it is converted into 5,6-epoxide, which is a powerful carcinogen (see De Robertis and De Robertis, Jr., 1987) A wide variety of drugs (e.g., Phenobarbital), when administrated to animals, they bring about the proliferation of the ER membranes (first RER and then SER) and /or enhanced activity of enzymes related to detoxification (Thorpoe, 1984). C. Functions of Rough Endoplasmic Reticulum The major function of the rough ER is the synthesis of protein. It has long been assumed that proteins destined for secretion (i.e., export) from the cell or proteins to be used in the synthesis of cellular membranes are synthesized on rough DR-bound ribosomes, while cytoplasmic proteins are translated for the most part on free ribosomes. In fact, the array of the rough endoplasmic reticulum provides extensive surface area for the association of metabolically active enzymes, amino acids and ribosomes. There is more efficient functioning of these materials to synthesize proteins when oriented on a membrane surface than when they are simply in solution, mainly because 55 chemical combinations between molecules can be accomplished in specific geometric patterns. The membrane-bound ribosomes are attached with specific binding sites or receptors of rough ER membrane by their large 60S subunit, with small or 40S subunit sitting on top like a cap. These receptors are membrane proteins which extend well into and possibly through the lipid bilayer. The receptor proteins with bound ribosomes can float laterally like other membrane proteins and may facilitate formation of the polysome and probably translation which requires that mRNA and ribosome move with respect to each other. Further, the secretory proteins, instead of passing into the cytoplasm, appear to pass instead into the cisternae of the rough ER and are, thus, protected 56 from protease enzymes of cytoplasm. It is calculated that about 40 amino acid residues long segment at the - COOH end of the nascent protein remains protected inside the tunnel of 'free' or 'bond' ribosomes and rest of the chain, with-NH2 end, is protected by the lumen of RER. The passage of nascent polypeptide chain into the ER cisterna take place during translation leaving only a small segment exposed to the cytoplasm at any one time. Protein glycosylation. The covalent addition of sugars to the secretory proteins (i.e., glycosylation) is one of the major biosynthetic functions of rough ER. Most of the proteins that are isolated in the lumen of RER before being transported to the Golgi apparatus, lysosomes, plasma membrane or extracellular space, are glycoproteins (A notable exception is albumin). In contrast, very few proteins in the cytosol (Cytoplasmic matrix) are glycosylated and those that carry them have a different sugar modification. The process of protein glycosylation in RER lumen is one of the most well understood cell biological phenomena. During this process, a single species of loligosaccharide (Which comprises N-acetyl-glucosamine, mannose and glucose, containing a total of 14 sugar residues) is transferred to proteins in the ER. Check your progress: 1 1. Notes- Write your answer in the space given. 2. Compare your answer with the one given at the end of the unit. 1. What is photo-respiration? 2. Explain Glycogenolysis? ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 57 ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 5.6 Techniques in cell biology; Immunotechniques Radioimmunoassay On of the most sensitive techniques for detecting antigen or antibody is radioimmunoassay (RIA). The technique was first developed in 1960 by two endocrinologists, S.A. Berson and Rosalyn Yalow, to determine levels of insulin-antiinsulin complexes in diabetics. Although their technique encountered some skepticism, it soon proved its value for measuring hormones, serum proteins, drugs, and vitamins at concentrations of 0.001 micrograms per milliliter or less. In 1977, some years after Berson's death, the significance of the technique was acknowledged by the award of a Nobel Prize to Yalow. The Principle of RIA involves competitive binding of radiolabeled antigen and unlabeled antigen to a high-affinity antibody. The labeled antigen is mixed with antibody at a concentration that saturates the antigen-binding sites of the antibody. Then test samples of unlabeled antigen of unknown concentration are added in progressively larger amounts. The antibody does not distinguish labeled from unlabeled antigen, so the two kinds of antigen compete for available binding sites on the antibody. As the concentration of unlabeled antigen increases, more labeled antigen will be displaced from the binding sites. The decrease in the amount of radio labeled antigen bound to specific antibody in the presence of the test sample is measured in order to determine the amount of antigen present in the test sample. The antigen is generally labeled with a gamma-emitting isotope such as 125I, but beta-emitting isotopes such as tritium (3H) are also routinely used as labels. The radio labeled antigen is part of the assay mixture; the test sample may be a complex mixture, such as serum or other body fluids, that contains the unlabeled antigen. The first step in setting up an RIA is to determine the amount of antibody needed to bind 50% to 70% of a fixed quantity of radioactive antigen in the assay mixture. This ratio of antibody to Ag* is chosen to ensure that the number of epitope presented by the labeled antigen always exceeds the total number of antibody binding sites. 58 Consequently, unlabeled antigen added to the sample mixture will complete with radio labeled antigen for the limited supply of antibody. Even a small amount of unlabeled antigen added to the assay mixture of labeled antigen and antibody will cause a decrease in the amount of radioactive antigen bound, and this decrease will be proportional to the amount of unlabeled antigen added. To determine the amount of labeled antigen bound, the Ag-Ab complex is precipitated to separate it from free antigen (antigen not bound to antibody), and the radioactivity in the precipitate is measured. A standard curve can be generated using unlabeled antigen samples of known concentration (in place of the test sample), and from this plot the amount of antigen in the test mixture may be precisely determined. Several methods have been developed for separating bound antigen from free antigen in RIA. One method involves precipitating Ag-Ab complexes with a secondary anti-isotope antiserum. For example, if the Ag-Ab complex contains rabbit IgG antibody, then goat anti-rabbit IgG will bind to the rabbit IgG and precipitate the complex. Another method makes use of the fact that protein A of Staphylococcus aureus has high affinity for IgG. If the Ag-Ab complex contains an IgG antibody, the complex can be precipitated by mixing with formalin-killed S. aureus. After removal of the complex by either of these methods, the amount of free labeled antigen remaining in the supernatant can be measured in a radiation counter; subtracting this value from the total amount of labeled antigen added yields the amount of labeled antigen bound. Various solid-phase RIAs have been developed that make it easier to separate the Ag-Ab complex from the unbound antigen. In some cases, the antibody is covalently cross-linked to Sepharose beads. The amount of radiolabeled antigen bound to the beads can be measured after the beads have been centrifuged and washed. Alternatively, the antibody can be immobilized on polystyrene or poly-vinylchloride wells and the amount of free labeled antigen in the supernatant can be determined in a radiation counter. In another approach, the antibody is immobilized on the walls of microtiter wells and the amount of bound antigen determined. Because the procedure requires only small amounts of sample and can be conducted in small 96well microtiter plates (slightly larger than a 3 × 5 card), this procedure is well suited for determining the concentration of a particular antigen in large numbers of samples. For example, a microtiter RIA has been widely used to screen for donor blood has sharply reduced the incidence of hepatitis B infections in recipients of blood transfusions. 59 ELISA Enzyme-Linked Immunosorbent assay, commonly known as ELISA (or EIA), is similar in principle to RIA but depends on an enzyme rather than a radioactive label. An enzyme conjugated with an antibody reacts with a colorless substrate to generate a colored reaction product. Such a substrate is called a chromogenic substrate. A number of enzymes have been employed for ELISA, including alkaline phosphatase, horseradish peroxidase, and -galactosidase. These assays match the sensitivity of RIAs and have the advantage of beings safer and less costly. There are numerous variants of ELISA A number of variations of ELISA have been developed, allowing qualitative detection or quantitative measurement of either antigen or antibody. Each type of ELISA can be used qualitatively to detect the presence of antibody or antigen. Alternatively, a standard curve based on known concentrations of antibody or antigen is prepared, from which the unknown concentration of a sample can be determined. Indirect ELISA Antibody can be detected or quantitatively determined with an indirect ELISA. Serum or some other sample containing primary antibody (Ab 1) is added to an antigen coated microtiler well and allowed to react with the antigen attached to the well. After any free Ab1 is washed away, the presence of antibody bound to the antigen is detected by adding an enzyme-conjugated secondary antibody (Ab2) that binds to 60 the primary antibody. Any free Ab2 is then washed away, and a substrate for the enzyme is added. The amount of colored reaction product that forms is measured by specialized spectrophotometric plate readers, which can measured the absorbance of all of the wells of a 96-well plate in seconds. Indirect ELISA is the method of choice to detect the presence of serum antibodies against human immunodeficiency virus (HIV), the causative agent of AIDS. In this assay, recombinant envelope and core proteins of HIV are adsorbed as solid-phase antigens to microtiter wells. Individuals infected with HIV will produce serum antibodies to epitopes on these viral proteins. Generally, serum antibodies to HIV can be detected by indirect ELISA. Sandwich ELISA Antigen can be detected or measured by a sandwich ELISA. In this technique, the antibody (rather than the antigen) is immobilized on a microtiter well. A sample containing antigen is added and allowed to react with the immobilized antibody. After the well is washed, a second enzyme-linked antibody specific for a different epitope 61 on the antigen is added and allowed to react with the bound antigen. After any free second antibody is removed by washing substrate is added, and the colored reaction product is measured. Competitive ELISA Another variation for measuring amounts of antigen is competitive ELISA. In this technique, antibody is first incubated in solution with a sample containing antigen. The antigen antibody mixture is then added to an antigen coated microtiter well. The more antigen present in the sample, the less free antibody will be available to bind to the antigen-coated well. Addition of an enzyme-conjugated secondary antibody (Ab2) specific for the isotype of the primary antibody can be used to determine the amount of primary antibody bound to the well, as in an indirect ELISA. In the competitive assay, however, then higher the concentration of antigen in the original sample, the lower the absorbance. Western Blotting Identification of a specific protein in a complex mixture of proteins can be a accomplished by a technique known as Western blotting, named for its similarity to Southern blotting, which detects DNA fragments, and Northern blotting which detects mRNA. In Western, a protein mixture is electrophoretically separated on an SDSpolyacrylamide gel (SDS-PAGE), a slab gel infused with sodium dodecyl sulfate (SDS), a dissociating agent. The protein bands are transferred to a nitrocellulose membrane by electrophoresis, and the individual protein bands identified by flooding the membrane with radiolabeled or enzyme-protein of interest. The Ag-Ab complexes that form on the band containing the protein recognized by the antibody can was bound by a radioactive antibody, its position on the blot can be determined by exposing the membrane to a sheet of x-ray film, a procedure called autoradiography. However, the linked antibodies against the protein. After binding of the enzyme antibody conjugate, addition of a chromogenic substrate that produces a highly colored and insoluble product causes the appearance of a colored band at the site of the target antigen. Even greater sensitivity can be achieved if a chemicluminescent compound with suitable enhancing agents is used to produce light at the antigen site. Western blotting can also identify a specific antibody in a mixture. In this case, known antigens of well defined molecular weight are separated by SDS-PAGE and blotted onto nitrocellulose. The separated bands of known antigens are then probed 62 with the sample suspected of containing antibody specific for one or more of these antigens. Reaction of an antibody with a band is detected by using either radiolabeled or enzyme-linked secondary antibody that is specific for the spiciest of the antibodies in the test sample. The most widely used application of this procedure is in confirmatory testing for HIV, where western blotting is used to determine whether the patient has antibodies that react with one or more viral proteins. 5.7 FISH-Fluorescent in situ hybridization FISH (Fluorescent in situ hybridization) is a cytogenetic technique that can be used to detect and localize the presence or absence of specific DNA sequences on chromosomes. It uses fluorescent probes that bind to only those parts of the chromosome with which they show a high degree of sequence similarity. Fluorescence microscopy can be used to find out where the fluorescent probe bound to the chromosome. FISH is often used for finding specific features in DNA. These features can be used in genetic counseling, medicine, and species identification. Probes are often derived from fragments of DNA that were isolated, purified, and amplified for use in the Human Genome Project. The size of the human genome is so large, compared to the length that could be sequenced directly, that it was necessary to divide the genome into fragments. The fragments were added into a framework that made it possible to use bacteria to replicate the fragments. The fragments were put into order by analyzing size-exclusion separation of enzymatically-digested fragments. Clonal populations of bacteria, each population maintaining a single artificial chromosome, are stored in various laboratories around the world. The artificial chromosomes (BAC) can be grown, extracted, and labeled, in any lab. These fragments are on the order of 100 thousand base-pairs, and are the basis for most FISH probes. Preparation and Hybridization Process 63 Scheme of the principle of the FISH Experiment to localize a gene in the nucleus. First, a probe is constructed. The probe must be large enough to hybridize specifically with its target but not too large to impede the hybridization process. The probe is tagged directly with fluorophores, with targets for antibodies or with biotin. Tagging can be done in various ways, for example nick translation and PCR using tagged nucleotides. Then, an interphase or metaphase chromosome preparation is produced. The chromosomes are firmly attached to a substrate, usually glass. Repetitive DNA sequences must be blocked 64 by adding short fragments of DNA to the sample. The probe is then applied to the chromosome DNA and incubated for approximately 12 hours while hybridizing. Several wash steps remove all un-hybridized or partially-hybridized probes. The results are then visualized and quantified using a microscope that is capable of exciting the dye and recording images. If the fluorescent signal is weak, amplification of the signal may be necessary in order to exceed the detection threshold of the microscope. Florescent signal strength depends on many factors such as probe labeling efficiency, the type of probe, and the type of dye. Fluorescently-tagged antibodies or streptavidin are bound to the dye molecule. These secondary components are selected so that they have a strong signal. Variations on Probes and Analysis FISH is a very general technique. It is often arbitrarily divided into more specific categories based on application, however each category is similar in that, in a chemical sense, the technique is the same; hybridization is the common denominator. The differences between the various FISH techniques are usually due to the construction and content of the fluorescently-labeled DNA probe. The size, overlap, colour, and mixture of the probes make possible all FISH techniques. Probe size is important because longer probes hybridize more specifically than shorter probes. The overlap defines the resolution of detectable features. If the goal of an experiment is to detect the breakpoint of a translocation, then the overlap of the probes — the degree to which one DNA sequence is contained in the adjacent probes — defines the minimum window in which the breakpoint occurs. The mixture of probes determines the type of feature the probe can detect. Probes that hybridize along an entire chromosome are used to count the number of a certain chromosome, show translocations, or identify extra-chromosomal fragments of chromatin. This is often called "whole-chromosome painting." If every possible probe is used, every chromosome, (in essence the whole genome) would be marked fluorescently, which would not be particularly useful for determining features of individual sequences. A mixture of smaller probes can be created that are specific to a particular region (locus) of DNA; these mixtures are used to detect deletion mutations. When combined with a specific colour, a locus-specific probe mixture is used to detect very specific translocations. Special locusspecific probe mixtures are often used to count chromosomes, by binding to the centromeric 65 regions of chromosomes, which are unique enough to identify each chromosome (with the exception of Chromosome 13, 14 21, 22.) Because modern microscopes can detect a range of colours in fluorescent dyes each human chromosome can be identified (M-FISH) using whole-chromosome probe mixtures and a variety of colours. There are currently twice as many chromosomes than fluorescent dye colours. However, ratios of probe mixtures can be used to create additional colours. As with comparative genomic hybridization, the probe mixture for the secondary colours is created by mixing the correct ratio of two sets of differently-labeled probes for the same chromosome. Differently coloured probes can be used for the detection of translocations. Several techniques exploit the resolution limitations of microscopes to resolve spatial distributions of dye below a few hundred nanometers. Colours that are adjacent appear to overlap, and a secondary colour is observed. In reciprocal translocations, where both breakpoints are known, locus-specific probes are made for it and part of the region one either side of breakpoint. In normal cells, two colours will be visible; in diseased cells such as those found in BCR/ABL translocations, the two dye colours overlap, and a third colour is observed. This technique is known as double-fusion FISH or D-FISH. In translocations where only one of the breakpoints is known or constant, locus-specific probes are made for one side of the breakpoint and the other intact chromosome. In normal cells, the secondary colour is observed, but only the primary colour is observed when the translocation occurs. This technique is known as "break-apart FISH". Medical applications Often parents of children with a developmental delay want to know more about their child's conditions before choosing to have another child. These concerns can be addressed by analysis of the parents' and child's DNA. In cases where the child's developmental delay is not understood, the cause of it can be determined using FISH and cytogenetic techniques. Examples of diseases that are diagnosed using FISH include Prader-Willi syndrome, Angelman syndrome, 22q13 deletion syndrome, chronic myelogenous leukemia, acute lymphoblastic leukemia, Cri-du-chat, Velocardiofacial syndrome, and Down syndrome. In medicine, FISH can be used to form a diagnosis, to evaluate prognosis, or to evaluate remission of a disease, such as cancer. Treatment can then be specifically tailored. A traditional exam involving metaphase chromosome analysis is often unable to identify features that distinguish one disease from another, due to subtle chromosomal features; FISH 66 can elucidate these differences. FISH can also be used to detect diseased cells more easily than standard Cytogenetic methods, which require dividing cells and requires labor and timeintensive manual preparation and analysis of the slides by a technologist. FISH, on the other hand, does not require living cells and can be quantified automatically, a computer counts the fluorescent dots present. However, a trained technologist is required to distinguish subtle differences in banding patterns on bent and twisted metaphase chromosomes. Species identification FISH is often used in clinical studies. If a patient is infected with a suspected pathogen, bacteria, from the patient's tissues or fluids, are typically grown on agar to determine the identity of the pathogen. Many bacteria, however, even well-known species, do not grow well under laboratory conditions. FISH can be used to detect directly the presence of the suspect on small samples of patient's tissue. FISH can also be to used compare the genomes of two biological species, to deduce evolutionary relationships. A similar hybridization technique is called a zoo blot. Bacterial FISH probes are often primers for the 16s rRNA region. FISH is widely used in the field of microbial ecology, to identify microorganisms. Bio-films, for example, are composed of complex (often) multi-species bacterial organizations. Preparing DNA probes for one species and performing FISH with this probe allows one to visualize the distribution of this specific species within the bio-film. Preparing probes (in two different colors) for two species allows to visualize/study co-localization of these two species in the bio-film, and can be useful in determining the fine architecture of the bio-film. 5.8 Confocal Microscopy In a conventional (i.e., wide-field) fluorescence microscope, the entire specimen is flooded in light from a light source. Due to the conservation of light intensity transportation, all parts of the specimen throughout the optical path will be excited and the fluorescence detected by a photo-detector or a camera. In contrast, a confocal microscope uses point illumination and a pinhole in an optically conjugate plane in front of the detector to eliminate out-of-focus information. Only the light within the focal plane can be detected, so the image quality is much better than that of wide-field images. As only one point is illuminated at a time in 67 confocal microscopy, 2D or 3D imaging requires scanning over a regular raster (i.e. a rectangular pattern of parallel scanning lines) in the specimen. The thickness of the focal plane is defined mostly by the square of the numerical aperture of the objective lens, and also by the optical properties of the specimen and the ambient index of refraction. Types Three types of confocal microscopes are commercially available: Confocal laser scanning microscopes, spinning-disk (Nipkow disk) confocal microscopes and Programmable Array Microscopes (PAM). Confocal laser scanning microscopy yields better image quality than Nipkow and PAM, but the imaging frame rate was very slow (less than 3 frames/second) until recently; spinning-disk confocal microscopes can achieve video rate imaging—a desirable feature for dynamic observations such as live cell imaging. Confocal laser scanning microscopy has now been improved to provide better than video rate (60 frames/second) imaging by using MEMS based scanning mirrorsConfocal microscopy offers several advantages over conventional optical microscopy, including controllable depth of field, the elimination of image degrading out-of-focus information, and the ability to collect serial optical sections from thick specimens. The key to the confocal approach is the use of spatial filtering to eliminate out-of-focus light or flare in specimens that are thicker than the plane of focus. There has been a tremendous explosion in the popularity of confocal microscopy in recent years, due in part to the relative ease with which extremely high-quality images can be 68 obtained from specimens prepared for conventional optical microscopy, and in its great number of applications in many areas of current research interest. Basic Concepts – Current instruments are highly evolved from the earliest versions, but the principle of confocal imaging advanced by Marvin Minsky, and patented in 1957, is employed in all modern confocal microscopes. In a conventional wide field microscope, the entire specimen is bathed in light from a mercury or xenon source, and the image can be viewed directly by eye or projected onto an image capture device or photographic film. In contrast, the method of image formation in a confocal microscope is fundamentally different. Illumination is achieved by scanning one or more focused beams of light, usually from a laser or arcdischarge source, across the specimen. This point of illumination is brought to focus in the specimen by the objective lens, and laterally scanned using some form of scanning device under computer control. The sequences of points of light from the specimen are detected by a photomultiplier tube (PMT) through a pinhole (or in some cases, a slit), and the output from the PMT is built into an image and displayed by the computer. Although unstained specimens can be viewed using light reflected back from the specimen, they usually are labeled with one or more fluorescent probes. Imaging Modes - A number of different imaging modes are used in the application of confocal microscopy to a vast variety of specimen types. They all rely on the ability of the technique to produce high-resolution images, termed optical sections, in sequence through relatively thick sections or whole-mount specimens. Based on the optical section as the basic image unit, data can be collected from fixed and stained specimens in single, double, triple, or multiple-wavelength illumination modes, and the images collected with the various illumination and labeling strategies will be in register with each other. Live cell imaging and time-lapse sequences are possible, and digital image processing methods applied to sequences of images allow z-series and three-dimensional representation of specimens, as well as the time-sequence presentation of 3D data as four-dimensional imaging. Reflected light imaging was the mode used in early confocal instruments, but any of the transmitted light imaging modes commonly employed in microscopy can be utilized in the laser scanning confocal microscope. 69 Refinements in design have simplified confocal microscopy to the extent that it has become a standard research tool in cell biology. However, as confocal microscopes have become more powerful, they have also become more demanding of their optical components. In fact, optical aberrations that cause subtle defects in image quality in wide field microscopy can have devastating effects in confocal microscopy. Unfortunately, the exacting optical requirements of confocal microscopy are often hidden by the optical system that guarantees a sharp image, even when the microscope is performing poorly. Three-Color Imaging for Confocal Microscopy - The laser scanning confocal microscope (LSCM) is routinely used to produce digital images of single-, double-, and triple-labeled fluorescent samples. The use of red, green and blue (RGB) color is most informative for displaying the distribution of up to three fluorescent probes labeling a cell, where any colocalization is observed as a different additive color when the images are colorized and merged into a single three-color image. Check your progress: 2 1. Notes- Write your answer in the space given. 2. Compare your answer with the one given at the end of the unit. 1. What is FISH? 2. What are the three types of confocal microscopes? -----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------5.9 Let us sum up Microbodies are spherical or oblate in form. They are bounded by a single membrane and have an interior or matrix which is amorphous or granular Microcopies are most easily distinguished from other cell organelles by their content of catalase enzyme. 70 In green leaves, there are peroxisomes that carry out a process called photorespiration which is a light - stimulated production of CO2 that is different from the generation of CO2 by mitochondria in the dark. The Golgi apparatus occurs in all cells except the prokaryotic cells (viz., mycoplasmas, bacteria and blue green algae) and eukaryotic cells of certain fungi, sperm cells of bryophytes and pteridiophytes, cells of mature sieve tubes of plants and mature sperm and red blood cells of animals. Their number per plant cell can vary from several hundred as in tissues of corn root and algal rhizoids (i.e., more than 25,000 in algal rhizoids, Sievers, 1965), to a single organelle in some algae. The lysosomes (Gr. lyso=digestive + soma = boy) are tiny membrane-bound vesicles involved in intracellular digestion. They contain a variety of hydrolytic enzymes that remain active under acidic conditions. The lysosomal lumen is maintained at an acidic pH (around 5) by an ATP-driven proton pump in the membrane. Thus, these remarkable organelles are primarily meant for the digestion of a variety of biological materials and secondarily cause aging and death of animal cells and also a variety of human diseases such as cancer, gout Pompe's silicosis and I-cell disease. The endoplasmic reticulum provides and ultrastructural skeletal framework to the cell and gives mechanical support to the colloidal cytoplasmic matrix. The exchange of molecules by the process of osmosis, diffusion and active transport occurs through the membranes of endoplasmic reticulum. Like plasma membrane, the ER membrane has permeases and carries. The endoplasmic membranes contain many enzymes which perform various synthetic and metabolic activities. Further the endoplasmic reticulum provides increase surface for various enzymatic reactions. RIA involves competitive binding of radiolabeled antigen and unlabeled antigen to a high-affinity antibody. The labeled antigen is mixed with anti-body at a concentration that saturates the antigen-binding sites of the antibody. ELISA (or EIA), is similar in principle to RIA but depends on an enzyme rather than a radioactive label. An enzyme conjugated with an antibody reacts with a colorless substrate to generate a colored reaction product. Such a substrate is called a chromogenic substrate. A number of enzymes have been employed for 71 ELISA, including alkaline phosphatase, horseradish peroxidase, and galactosidase. These assays match the sensitivity of RIAs and have the advantage of beings safer and less costly. FISH (Fluorescent in situ hybridization) is a cytogenetic technique that can be used to detect and localize the presence or absence of specific DNA sequences on chromosomes. A confocal microscope uses point illumination and a pinhole in an optically conjugate plane in front of the detector to eliminate out-of-focus information. Only the light within the focal plane can be detected, so the image quality is much better than that of wide-field images. As only one point is illuminated at a time in confocal microscopy, 2D or 3D imaging requires scanning over a regular raster (i.e. a rectangular pattern of parallel scanning lines) in the specimen. Check your progress 1: The key 1. Photo-respiration is so called because light induces the synthesis of glycolic acid in chloroplasts. This process involves intervention of two organelles : chloroplasts and peroxisomes. 2. Glycogenolysis and blood glucose homeostasis. This process of glycogen synthesis (glycogenesis) occurs in the cytosol (in glycosomes). The enzyme UDPG-glycogen transferase, which is directly involved in the synthesis of glycogen by addition of uridine diphosphate glucose (UDPG) to primer glycogen is bound to the glycogen particles or glycosomes. Check your progress 2 : The key 1. FISH (Fluorescent in situ hybridization) is a cytogenetic technique that can be used to detect and localize the presence or absence of specific DNA sequences on chromosomes. 2. Three types of confocal microscopes are commercially available: Confocal laser scanning microscopes, spinning-disk (Nipkow disk) confocal microscopes and Programmable Array Microscopes (PAM). 5.10 Assignment/ Activity 1. Explain different molecular diagnostic techniques with their use. 2. Do a activity in sandwich ELISA Technique.(Use Kit from Gene if required.) 72 5.11 Reference Annelie Pernthaler, Jakob Pernthaler, Rudolf Amann (2002): Fluorescence In Situ Hybridization and Catalyzed Reporter Deposition for the Identification of Marine Bacteria, APPLIED AND ENVIRONMENTAL MICROBIOLOGY, June 2002, p. 3094–3101 Vol. 68, No. 6 DOI: 10.1128/AEM.68.6.3094–3101.2002 James B. Pawley - Department of Zoology, 1117 W. Johnson Dr., University of Wisconsin, Madison, Wisconsin 53706. Kenneth W. Dunn and Exing Wang - Department of Medicine, Indiana University, School of Medicine, 1120 South Drive, FH115, Indianapolis, Indiana 46202-5116. Matthew Parry-Hill, Thomas J. Fellers, and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310. Michael Wagner, Matthias Horny and Holger Daimsz (2003): Fluorescence in situ hybridisation for the identification and characterisation of prokaryotes. Current Opinion in Microbiology 2003, 6:302–309 DOI:10.1016/S1369-5274(03)00054-7 Stephen W. Paddock, Eric J. Hazen, and Peter J. DeVries - Laboratory of Molecular Biology, Howard Hughes Medical Institute, University of Wisconsin, Madison, Wisconsin 53706. V. J. Sieben, C. S. Debes-Marun, P. M. Pilarski, G. V. Kaigala, L. M. Pilarski, and C. Backhouse, "FISH and chips: chromosomal analysis on microfluidic platforms", IET Nanobiotechnology, vol. 1(3), pp. 27-35, June 2007. DOI:10.1049/iet-nbt:20060021 73 74