HLA-B27 unit dose single well 2014 49V R01
advertisement

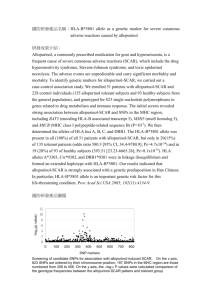
® October 2014 Rev. No: 01 HLA-B*27 – unit dose single well 101.911-96/96u 49V Visit www.olerup-ssp.com for “Instructions for Use” (IFU) and detailed Product Inserts Gel Documentation Form and Worksheet HLA-B*27 unit dose single well (101.911-96/96u) Lot: 49V Name:____________________ Test Date: ________ Sample ID:________________ Tested By: ____ Expiry Date: 2016-August-01 _ Review Date:_______________ _____ Reviewed By:_______________ DNA Conc.(ng/ul):_________ Gel Picture PHOTO DOCUMENT Well No. 1 ICB 430 Am pS 145 205 Well No. Specific Reaction 1 ‘ICB’ Internal Control Band, ‘AmpS’ Amplicon size Interpretation:_______________ Failed lanes: _________ Comments: ___________________ Notes: Product sizes are approximate. For detailed information, see the lot-specific Specificity Table and Interpretation Table. This table is intended as a guide. For interpretation always use the Interpretation Table and/or Specificity Table. Olerup SSP AB Franzengatan 5 SE-112 51 Stockholm Sweden For In Vitro Diagnostic Use Page 1 of 2 ® October 2014 Rev. No: 01 HLA-B*27 – unit dose single well 101.911-96/96u 49V Well No. 1 HLA-B allele 1,2 *27:01-27:05:22, 27:05:24-27:21, 27:2427:28, 27:30-27:74, 27:76, 27:78-27:84, 27:86-27:117 B*07:197, B*44:97, B*49:26 Well No. Visit www.olerup-ssp.com for “Instructions for Use” (IFU) and detailed Product Inserts 1 1 1 1The primers in this kit do not amplify the B*27:05:23, B*27:23, B*27:29, B*27:75, B*27:77 and B*27:85 alleles. Due to the sharing of sequence motifs between HLA-B alleles the B*07:197, B*44:97 and B*49:26 is amplified by primer mix 1. 2Alleles that have been deleted from or renamed in the official WHO HLA Nomenclature up to and including the last IMGT/HLA database release can be retrieved from web page http://hla.alleles.org/alleles/deleted.html. Change in revision R01 compared to R00: 1. The expiry date has been corrected. Olerup SSP AB Franzengatan 5 SE-112 51 Stockholm Sweden For In Vitro Diagnostic Use Page 2 of 2