Teachers Guide for Acid
advertisement

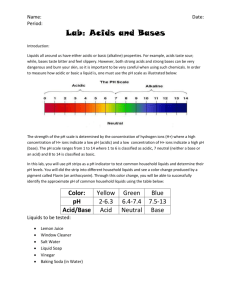
Teachers Guide for Acid-Base Reactions Many acids and bases can be found in your home. Acids are things that usually taste sour and sting when they get in your eye or a cut. Bases are things that usually feel slippery or taste bitter. Do not taste things in a lab because it could be dangerous. Indicators can be used to test whether a substance is an acid or a base. Indicators work by turning a certain color in an acid or base. In acid an indicator will turn red and in a base an indicator will turn blue. Litmus paper is special paper that has an indicator on it so that it will turn red or blue in acids or bases. When acids and bases mix together, a neutralization reaction occurs. After mixing an acid and base together the new product is not acidic or basic, it is neutral. In a neutralization reaction, a salt is formed. The acid-base reactions activity is a fun way for students to learn what household items are acidic and what are basic. They will learn about indicators and special paper with indicators on them called litmus paper. They will the finally learn what happens when you add acids and bases together to try and figure out what happened to a wool sweater (acidic fiber) when it got bleached (base). And how that is different from the cotton fabric (base) in the bleach (base) Materials (materials are enough for a group of 2-3 students) 14 containers or vials for holding liquids and solids labeled with what they are (10 for the younger kids) Lemon Juice Cranberry Juice Vinegar Baking Soda (3 vials with powdered baking soda and 1 with baking soda + water) Detergent( 3 vials with powdered detergent and 1 with detergent + water) Windex Egg Whites (older students only) Astringent (older students only) 6 cups for mixing Toothpicks for testing liquids Litmus paper or cabbage juice (directions are written for litmus paper) Piece of wool fabric Piece of Cotton Fabric Chlorine bleach 2 Glass jars Ahead of time place a piece of wool fabric in a glass jar with bleach, cover and set aside. After about five minutes, the fabric should begin to deteriorate because of an acid base reaction. Also put a piece of white cotton fabric in a jar of bleach and nothing should happen because cotton is a basic fabric. Story “I just got a new wool sweater from my mom for my birthday, and my sister was very jealous. It was this beautiful white wool sweater. I had spilled some mustard on it one day so I went down stairs to wash it with some bleach. I went upstairs to wash the dishes and when I came back down my new sweater was ruined. I think that when I was upstairs my sister went down and ruined it. What do you think?” or some version of the story. Next introduce the activities by describing acids, bases, indicators, litmus paper and for the older students neutralization reaction. All of the key words are defined on the student handouts. You can explain to the students that there are indicators that occur naturally like in red cabbages. You can make an indicator by boiling red cabbage in water for fifteen minutes and the juice can be used as an indicator. If you put a few drop of cabbage indicator in a base the solution will turn blue and if you put a few drops in an acid it will turn red. The students will then complete two activities. In the first activity, they will test the liquids to see if they are acidic or basic. The second activity, involves the student mixing powdered baking soda and detergent with acidic liquids (lemon juice, cranberry juice, vinegar, and astringent) and then with basic liquids (Windex and egg white). When mixed with acids, the detergent and baking soda should fizz with a color change when mixed with cranberry juice. When mixed with bases, nothing should happen. After completing the activity, ask the students if you should test the bleach to see if it is an acid or a base. The bleach is a base. Then tell them that you learned cotton is a basic fiber or is a base and wool is an acidic fiber or is an acid. Finally have them answer the rest of the questions on the work sheet. Mystery of the Wool Sweater Acid-Base Reactions Key words: acid, base, indicator Many acids and bases can be found in your home. Acids are things that usually taste sour and sting when they get in your eye or a cut. Bases are things that usually feel slippery or taste bitter. Do not taste things in a lab because it could be dangerous. Indicators can be used to see if something is an acid or a base. In an acid an indicator will turn red and in a base an indicator will turn blue. Directions: Separate the containers that have liquids and the containers that have powders. Put the powders to the side. Dip a toothpick into one of the liquids and then onto the litmus paper. Does it turn red or blue? If it’s red it is an acid. If it is blue it is a base. Circle red or blue. Lemon Juice Red Blue Cranberry Juice Red Blue Vinegar Red Blue Baking Soda Red Blue Detergent Windex Red Red Blue Blue When acids and bases mix together, reactions can happen. Sometimes they fizz and make new things. Sometimes they might change color. Now using the powders, mix together the things listed below in different cups and see what happens? Do they fizz? Do they change color? Baking Soda + Lemon Juice What happened? Baking Soda + Cranberry Juice What happened? Detergent + Vinegar What happened? Detergent +Windex What happened? Is the bleach red or blue? RED BLUE Is the bleach an acid or a base? ACID BASE What happened to the wool sweater? Mystery of the Wool Sweater Acid-Base Reactions Key words: acid, base, indicator, litmus paper, neutralization reaction Many acids and bases can be found in your home. Acids are things that usually taste sour and sting when they get in your eye or a cut. Bases are things that usually feel slippery or taste bitter. Do not taste things in a lab because it could be dangerous. Indicators can be used to test whether a substance is an acid or a base. Indicators work by turning a certain color in an acid or base. In acid an indicator will turn red and in a base an indicator will turn blue. Litmus paper is special paper that has an indicator on it so that it will turn red or blue in acids or bases. When acids and bases mix together, a neutralization reaction occurs. After mixing an acid and base together the new product is not acidic or basic, it is neutral. In a neutralization reaction, a salt is formed. Directions: Take out a piece of litmus paper. Using a toothpick dip it into one of the liquids and then onto the litmus paper. Record what color it turns and if it is an acid or a base. Put check marks in the appropriate boxes. Continue to follow this procedure until each of the liquids is tested. Record what each liquid is in the chart below and then separate them into groups. There are 6 vials with white powders in them. 3 of them are baking soda and 3 of them are detergent. In 4 separate cups pour two of the vials of detergent and two of the vials of baking soda. To each of these cups add one of each of the liquids from the opposite group and see what happens. Record your observations in the chart below. Pour the remaining baking soda and detergent in two more cups. Next pour in two liquids from the same group and see what happens. Record your observations in the chart below. Liquid Paper Paper Acid Base Turns Red Turns Blue Baking Soda Cranberry Juice Lemon Juice Egg Whites Vinegar Detergent Windex Astringent Adding liquids from the opposite group (fill in what liquid you added) Baking Soda + ____________________ What happened? Baking Soda + ____________________ What happened? Detergent + ____________________ What happened? Detergent + ____________________ What happened? Adding liquids from the same group (fill in what liquid you added) Baking Soda + ____________________ What happened? Detergent + ____________________ What happened? Is the bleach acidic or basic? ____________________ What happened to the wool sweater?