File - quest4confidence
advertisement

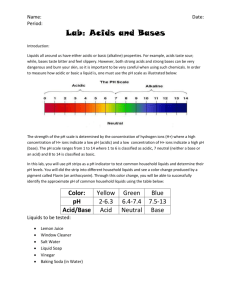
Acids and Bases What are acids and bases? Common acid Vinegar Tastes sour Common acid Baking soda Tastes bitter There are many kinds of acids and bases. Some strong, some weak, some can burn through your hands, some you can eat. So, what are acids and bases. The simple explanation is that an acid is a proton seller and a base is a proton eater. Proton for sale ! A Acid I want to eat a proton ! B Base I really want to eat a proton B Strong base Nah, I don’t feel hungry. B Weak base I really want to sell this proton ! + + I can sell it later. A + A + Strong acid Weak acid Here are a few acids and bases Acids, strongest to weakest Bases, weakest to strongest Sulfuric (H2SO4) Hydrochloric (HCl) Phosphoric (H3PO4) Acetic (vinegar) Carbonic (H2CO3) Acetate (CH3CO2-) Bicarbonate (baking soda) Ammonia (NH3) Carbonate (CO3-) Hydroxide (OH-) pH (Power of Hydrogen) There are strong acids and bases , and there are weak ones. But how do you measure acidity? Chemists use pH to measure it. pH ranges from 0 to 14. Water has a pH of 7, which is neutral. The lower the pH, the more acidic the solution is. The higher the pH, the more basic the solution is. Some acids and bases with their pH 0 1 2 3 4 5 6 7 Acids 5% sulfuric acid stomach acid lemons, vinegar apples, grapefruit, oranges, Coca-Cola tomatoes coffee, bread, potatoes, natural rivers pure water Bases pure water sea water, baking soda 7 8 9 10 water in Mono Lake, milk of magnesia 11 12 lime water 14 lye, 4% sodium hydroxide You can see that since 5% sulfuric acid has a pH of 0, highly concentrated sulfuric acid would have negative pH. Measuring pH Some dyes will show different colors under different pH values. The pH papers have dyes that show different colors in different pH values but most have a range. For example, take the picture right here. The range of this type it is 1 to 12. If the paper turns red, the pH would be 1, a strong acid. If the paper turns purple, the pH would be 12, a strong base. If the paper turns green, the pH would be 8, a weak base. . When acids and bases meet When a acid and base of equal strength meet, the acid gives of it’s proton and the base “eats it up.” With the acid’s proton and the base’s “hunger” gone, the solution becomes neutral. For example, when you mix HCl (hydrochloric acid) with NaOH (sodium hydroxide), the H, along with the OH, becomes water and the Na plus the Cl become NaCl (a.k.a. table salt) so the whole solution turns neutral. Here! Thanks! + Some common acid –base reactions There are many common acid –base reactions like when you rise bread. In baking powder there is sodium bicarbonate (baking soda) and an acid. When the baking powder is mixed with water and flour, the reaction between the acid and base will release carbon dioxide. When heated, the carbon dioxide expands, causing the bread rise. Coco-Cola uses a similar reaction. Other cartoon books from the author