sintering
advertisement

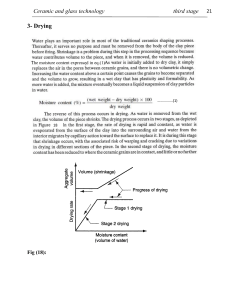
5. Sintering and Consolidation of Ceramics 5.1 Solid State Sintering 5.1.1. The Phenomenon of Sintering - Introduction What is Sintering ? Sintering commonly refers to processes involved in the heat treatment of powder compacts at elevated temperatures, usually at T > 0.5Tm [K], in the temperature range where diffusional mass transport is appreciable. Successful sintering usually results in a dense polycrystalline solid. However, sintering can proceed only locally (i.e. at contact point of grains), without any appreciable change in the average overall density of a powder compact. Basic Thermodynamics of Sintering The driving force for sintering is a decrease in the surface free energy of powdered compacts, by replacing solidvapour interfaces (of surface energy SV) with solid-solid (SS) interfaces, where SS < SV. Thermodynamically, then, sintering is an irreversible process in which a free energy decrease is brought about by a decrease in surface area. At a typical specific surface S of ceramic powders S = 1-10 m2/g and SV = 1 - 2 J/m2. The resulting excess of surface energy of SV = 1-20 J/g is small compared with the heat of chemical reactions (> 1 kJ/g) but still sufficient to drive the sintering processes. Some mechanisms can lead to a waste of sintering driving force without assisting densification. These are the grain coarsening mechanisms, which are driven by the same force as sintering. The change of system energy dE due to sintering is therefore composed of the increase due to the creation of new grain boundary areas, dASS > 0, and due to the annihilation of vapour-solid interfaces, dASV < 0. The necessary global thermodynamic condition for the sintering to proceed is: dE = SS dASS + SV dASV < 0 The sintering process will stop when dE = 0, i.e. SS dASS + SV dASV = 0 or SS / SV = - dASV / dASS The sintering progress can be represented by plotting the total free surface area ASV, vs the total surface area of the grain boundaries ASS. At the start of sintering, all surface area equals the free surface area, since no grain boundaries exist yet, ASV = ASV0 and ASS = 0 . As sintering proceeds, ASV decreases and ASS increases, in such a way that a monotonically decreasing curve is obtained with the slope - dASV / dASS . If, at any point during sintering, the value of the slope reaches SS / SV , the sintering must stop due to the above equilibrium condition. The principal objective of sintering is the elimination of porosity, i.e. a minimum ASV , possibly ASV =0 and ASS large, meaning little grain growth. It is thus desirable that the sintering stop condition is reached when the slope dASV / dASS close to zero, i.e. SS << SV. Through this type of thermodynamic considerations it is suggested that sintering can be encouraged through manipulation of the doping and/or the environment, so the surface energy is maximised. Note that the global decrease of system free energy is a necessary, but not sufficient, condition for sintering. The sintering process will proceed only if driven by the local differences in chemical potential due to differences in curvatures between the grain and the neck. Classes (Categories) of Sintering The majority of non-silicate ceramics are processed through high-temperature treatment and sintering of powder compacts with little (<2 vol%) or no liquid phases. This is defined as Solid State Sintering, with predominant mass transport (i.e. densification mechanism) through solid-state diffusion. However, most silica-containing ceramics, including traditional porcelains as well as advanced silicon nitride, sinter in the presence of viscous glass-type liquids, with predominant mass transport (i.e. densification mechanism) through viscous flow. This is defined as Viscous Sintering. If the liquid component of the sintering system has low viscosity (e.g. molten cobalt in the “classical” system of WC/Co) the process is defined as Liquid Sintering. In this system the predominant densification mechanism is through rearrangement of the solid particles “submerged” in and wetted by the low viscosity liquid, and through dissolution and re-precipitation of the solid. Why Ceramics have to be Sintered? Ceramic processing is based on the sintering of powder compacts rather than melting/solidification/cold working (characteristic for metals), because: ceramics melt at high temperatures as-solidified microstructures can not be modified through additional plastic deformation and recrystallisation due to brittleness of ceramics. the resulting coarse grains would act as fracture initiation sites. low thermal conductivities of ceramics (<30-50 W/mK), in contrast to high thermal conductivity of metals (in the range 50-300 W/mK) cause large temperature gradients, and thus thermal stress and shock in meltingsolidification of ceramics. However, some ceramic refractories , such as Al2O3, Al2O3/ZrO2, are manufactured through the melting / casting / solidification process. If made properly, they are superior to the sintered versions, and the processing costs of large, ~ 1 m tall blocks is lower. Why do we need Sintering? The principal goal of sintering is the reduction of compact porosity. Sometimes the initial spaces between compacted grains of ceramics are called “voids”, to differentiate them from the isolated spaces = pores, which occur in the final stages of sintering. The sintering process is usually accompanied by other changes within the material, some desirable and some undesirable. The largest changes occur in: - strength, elastic modulus - hardness, fracture toughness - electrical and thermal conductivity - permeability to gases and liquids - average grain number, size and shape - distribution of grain size and shape - average pore size and shape - distribution of pore size and shape - chemical composition and crystal structure Sintering is a widely used but very complex phenomenon. The fundamental mechanisms of sintering are still a matter of controversy. Experimental quantification of changes in pore fraction and geometry during sintering can be attempted by several techniques, such as: - dilatometry - resilience - gas absorption - porosimetry - indirect methods (e.g. hardness) - quantitative microscopy The description of the sintering process has been derived from model experiments (e.g. sintering of a few spheres) and by observing powdered compact behaviour at elevated temperatures. The following phenomena were observed and was modelling attempted: - increase of interparticle contact area with time - rounding-off of sharp angles and points of contact - in most cases, the approach of particle centres and overall densification - decrease in volume of interconnected pores - continuing isolation of pores - grain growth and decrease in volume of isolated pores The development of microstructure and densification during sintering is a direct consequence of mass transport through several possible paths (one of these paths is usually predominant at any given stage of sintering) : - GAS phase (evaporation/condensation) - LIQUID phase (solution/precipitation) - SOLID phase (lattice diffusion) - INTERFACES (surface diffusion, grain boundary diffusion) - VISCOUS or PLASTIC FLOW, under capillary pressure (internal) or externally applied pressure (pressure-sintering, hot-pressing, hot-isostatic-pressing) Since certain mechanisms of mass transport can be dominant in some systems, two broad categories of sintering are recognised: 1. Solid-state sintering, where all densification is achieved through changes in particle shape, without particle rearrangement or the presence of liquid. 2. Liquid-phase sintering, where some liquid that is present at sintering temperatures aids compaction. Grain rearrangement occurs in the initial stage followed by a solution-reprecipitation stage. Usually, the liquid amount is not sufficient to fill the green-state porosity in normal liquid-assisted sintering of ceramics. In many instances, supposedly Solid State Sintering proceeds in the presence of previously undetected (or transient) small amounts of liquid (perhaps introduced as impurities during the powder preparation stage, such as silicates in oxide ceramics Al2O3 , ZrO2). Simple model experiments with spheres or rods illustrate sintering phenomena, classified into three stages of sintering, as discussed below. 1. Initial stage of sintering, involving 1A. Local point of contact formation or "fusion", without shrinkage of compact. This is accompanied by smoothing of the free surface of particles. 1B. Neck formation at the contact point, with the resulting concave curvature n ( where n =1/rn ) at the neck, in contrast to the convex curvature on the particle surface of radius r, where r >> rn. The two radiuses of the neck curvature, rn and y, represent an experimental justification for the two-sphere model of sintering. The processes 1A and 1B result in densification of the sintering component by ~10%. That is, if the relative green density after forming of the particle compact was 60%, the density after initial stage would be about 70% of the theoretical density TD. However, the 10% densification in the initial stage is reached very quickly (seconds or minutes) after exposing powder to high temperature, because of the large surface area and the high driving force for sintering. 2. Intermediate stage of sintering, involving: 2A. neck growth, 2B. pores forming arrays of interconnected cylindrical channels 2C. particle centres approaching one another, with the resulting compact shrinkage. The shrinkage in the intermediate stage can result in additional densification by as much as 25%, or to a total of about 95% of the TD (theoretical density), compare. However, shrinkage does not necessarily have to take place during the intermediate stage of sintering. For example, shrinkage would not occur if matter was transported FROM the particle SURFACE, and proceeded through either gas, solid or along interface as surface diffusion. During sintering, if the only material transport mechanism originates on the surface of particles, no compact shrinkage takes place. In such a case a change of the shape and size of pores and particles is observed and commonly defined as grain growth or coarsening. Grain growth/coarsening depletes the system of surface energy and effectively STOPS the densification of the compact, even if mass transport mechanisms other than evaporation/condensation or surface diffusion become operational. At best, the coarsening process decreases the driving force for sintering and therefore the sintering rate, expressed as shrinkage versus time, decreases in the intermediate stage. Densification in the intermediate stage takes place only if: - mass is transported from the particle volume Or - mass is transported from the grain boundary And - proceeds through the solid as volume diffusion or along a boundary as grain boundary diffusion towards the neck forming between the sintering particles. The origin of mass transport during sintering determines the ability of the compact to shrink and eliminate porosity. There are systems where sintering proceeds vigorously through evaporation/condensation or through surface diffusion, without any shrinkage of the compact. In most cases this is an undesirable phenomenon, whenever residual porosity is undesirable. In some systems (e.g. recrystallised SiC ) this technique of bonding ceramic particles is acceptable. The very same driving force which propels sintering (i.e. elimination of excessive surface energy) also leads to grain growth during sintering. In this process larger particles grow at the cost of smaller particles, according to the general formula: rn - r0n = k SV t where: r = average particle size after time t , r0 = initial average particle size at t = 0 , SV = surface energy, k,n = constants, n = 3 to 4 . The growth of large grains at the expense of small grains is defined as Ostwald ripening, with the grain size t1/3. 3. Final stage of sintering, involving: 3A. Isolation of pores, i.e. relative density exceeding ~93% 3B. Elimination of porosity 3C. Grain growth The final sintering stage begins at about 93-95% of theoretical density, when porosity is already isolated. Ideally, at the end of this stage all porosity is eliminated. The complete elimination of porosity in the final stage of sintering can only happen when all pores are connected to fast, short diffusion paths along grain boundaries (or, equivalently, if the grain boundaries remain attached to the pores). This favourable situation happens only if the pores follow the movement of the grain boundaries and are not trapped within grains. This means that discontinuous grain growth (i.e. few grains growing at a very large rate at the expense of all other grains, trapping porosity on its path) is suppressed through grain growth limiting additives, such as secondary phase particles at grain boundaries, and/or appropriate time and temperature control of the sintering process In the final stage of sintering small pores attached to grain boundaries move quickly to collapse together and thus reduce surface energy. Along with pores, grain boundary movement is accelerated, leading eventually to discontinuous grain growth, when pores do not pin any more the grain boundaries. This leads to rapidly moving grain boundaries that consume small grains. Thus, some grains appear with many highly curved sides and therefore grain boundary motion further accelerates, leaving behind closed porosity that is trapped within large grains. Closed porosity is difficult to eliminate, because it involves relatively slow diffusion through lattice of the gas trapped in the pore. The critical grain size rc at which discontinuous grain growth starts can be expressed as rc = rp/f , where rp and f are the average pore radius and pore volume fraction respectively. Pores trapped within large grains are much more difficult to eliminate in the final sintering stage, because diffusion paths are long and slow, because lattice diffusion is the controlling step. Moreover, trapped gas resists densification. The closed porosity at the final sintering stage allows external gas pressure to be applied isostatically in order to accelerate the final densification. This approach is commercially known as Gas Pressure Sintering (GPS) The principal variables that control shrinkage at all stages of sintering are: temperature; time; particle size; chemical composition of ceramic; and chemical composition and pressure of the gas phase Empirical Modeling of Sintering A large number of equations describing the variation of porosity P/P0 (or shrinkage L/L0) versus time t have been proposed. All of these equations use simplified assumptions of idealised models, or are based purely on empirical observations. These equations have usually forms of: P/P0 = (a + bt)c or P - P0 = dln(t/t0) or (L/L0 )n = at, where: a,b,c,n = constants. A remarkable feature of most of these equations is that, using curve-fitting procedures, a nearly perfect fit can be obtained with experimental data on sintering. However, little or no insight into sintering mechanisms is obtained and incorrect conclusions are drawn when physical meanings are attributed to the fitted constants. This is because real systems deviate significantly from idealised models: particles are irregular in size and shape particles are non-uniformly distributed in space and rearrange during sintering necks grow asymmetrically different sintering stages (neck formation, neck growth, rearrangement, grain growth with or without shrinkage, closed porosity sintering) are controlled by different mechanisms which interact and overlap in a complex way 5.1.2. Mechanisms of Mass Transport and Neck Growth in Solid State Sintering A macroscopically measured quantity (for example by dilatometry) indicative of densification during sintering is the compact shrinkage L/L. For the idealised model of a dense uniform arrangement of spherical particles of radius r, the relative centres approach by h/r, which is equal to the overall shrinkage of the compact L / L. In real systems, these quantities are generally not equal, because of particle shift and rearrangement, formation and break-up of contacts, and interactions with porosity. The mass transport from surfaces: evaporation-condensation The mass transport can proceed through a gas phase, driven by a differential in vapour pressure. Evaporation condensation takes place because the vapour pressure p1 on a curved surface of radius r1 is different from that on a flat surface (p0 , r0 = ) or any other surface at r2 . This is expressed by the Kelvin equation: ln (p1/p2) = ( SV / RT) (1/r1 + 1/r2) where = the molar volume of the species. For the simple case of r2 = r0 = and p2 = p0 (that is, for a flat surface), the above equation simplifies to: ln (p1/p2) (p1-p0)/p0 = p/p0 so p/p0 = ( SV)/(R T r1) Accordingly, convex surfaces show overpressure (r1 > 0, p > 0), whereas concave surfaces show underpressure (r1 < 0, p < 0). The pressure gradient results in faster evaporation from convex surfaces (i.e. from grains, especially small grains), transport under the pressure gradient, and condensation within concave surfaces (i.e. within necks). Quantitatively, the effect is not large. For example p/p0 = 1.02 for SiO2 of r1 = 10-7 m (0.1 m) at 1700C. The most important case of evaporation-condensation mass transport is from the surface of convex (spherical) particles (r > 0) to the surface of concave necks (rn < 0) where |rn| << |r| at the contact region. No shrinkage occurs in this process and y3 is a linear function of time: y3 = 2 t and h/r = L/L = 0 This type of transport dominates at the relatively high vapour pressures of 10-4 atmospheres, found for halides (e.g. NaCl at 700C). The log-log plot of the neck size versus time has a slope close to 1/3. The evaporation condensation process is suspected to stop the sinterability of some nonoxide ceramics (such as SiC, B 4C) at very high temperatures > 2100C. The model for mass transport from surfaces: surface or volume diffusion from surface Similar to evaporation/condensation, no shrinkage results from the other two mechanisms of mass transport from particle surfaces to necks through the two possible diffusion paths. (I) volume diffusion from particle surfaces to necks: y4 = 3 t and h/r = L/L = 0 (II) surface diffusion, along particle surfaces to necks: y7 = 4 t and h/r = L/L = 0 The model for mass transport from within the particle volume or from the grain boundary For mass transfer originating in the particle volume or at the grain boundary, the particle centres approach (h > 0) and shrinkage takes place. (I) for volume diffusion from grain boundary: y5 = 5 t and thus (h/r)2.5 = (L/L)2.5 = 5 t (II) for grain boundary diffusion from grain boundary: y6 = 6 t and thus (h/r)3 = (L/L)3 = 6 t Knowing the relationships of the type yn = t for different mass transport mechanisms, model experiments can be performed on presumably ideal compacts of spheres, according to the following plan: - measure compact shrinkage s = L/L versus time t: s1 at t1, s2 at t2 , etc. - plot log s versus log t, determine slope n , and find equation sn = t - compare the calculated n with the values characteristic for various mass transfer processes and determine the sintering mechanism. If the sintering mechanism is known, the optimum sintering procedures can be proposed. - knowing the average initial particle radius r, calculate the neck radius y at different times As h = y2/4r, then y(t) = 2 r ( t)1/2n. Similar calculations can be performed if the neck radius y(t) is measured directly. For diffusion-controlled mechanisms, i and i are proportional to the diffusion coefficient D = D0exp(-H/RT), where H is the activation energy for diffusion. For viscous flow sintering i and I are inversely proportional to the viscosity coefficient , where = 0exp(Q/RT) and Q is the activation energy for viscous flow. NOTE, that temperature has an exponential effect on the two above modes of sintering. Conversely, for evaporation-condensation, is proportional to vapour pressure and therefore is linear with temperature (pV=RT). It is therefore frequently found, that the detrimental evaporation-condensation mechanisms are predominant at lower temperatures, whereas viscous flow and/or diffusional sintering take over at higher temperatures. If it is desired to avoid substantial particles growth through evaporation-condensation before densification (i.e. in most cases of sintering), fast heating rate is recommended. Sintering kinetics is accelerated for 1. fine grained materials; 2. elevated temperatures 3. high surface energy The powder composition and sintering atmosphere intervene in the densification through modification of the diffusion coefficient D. However, increasing the temperature far beyond T = 0.5Tm does not guarantee an increased sintering rate and full densification. This is due to the coarsening of grains (as explained above, through evaporation/condensation and surface diffusion) that can effectively stop densification (compaction through volume diffusion and grain boundary diffusion). 5.1.3. Examples of Solid State Sintering Since ceramics are composed of at least two elements (typically oxygen or nitrogen and metal ions) BOTH ionic species must diffuse together to maintain the electrical neutrality of the system. Therefore, it is the diffusion coefficient of the slowest moving ion along its fastest path that controls mass transfer, and therefore densification during solid state sintering. To enhance sintering, the slowest moving ion must be identified and its diffusion along the fastest path should be "encouraged" through : chemical doping, atmospheric control an appropriate time/temperature cycle Sintering of MgO For example, MgO sintering proceeds through lattice diffusion. Diffusion of magnesium is faster than oxygen , DMg > DO. The sintering rate is increased if the diffusivity of oxygen is increased through the creation of additional oxygen vacancies through doping with M2O (for example Na2O). Sintering of Al2O3 Another example of solid state sintering is the sintering of Al2O3. Initially, it was observed, that small additives of MgO (0.25 wt%) in Al2O3 allow the achievement of fine-grained material at full density (which is the ceramist’s dream). Additional microstructural observations revealed that MgO eliminates the discontinuous grain growth of Al2O3 . It was found that grain boundaries do not break away from the pores, which prevents the inclusion of pores trapped inside new large grains, with slow/long diffusion paths for densification. The mechanism by which MgO slows down grain boundary movement in alumina could be as follows: - The majority of MgO doped into Al2O3 resides at the grain boundaries, because the dissolution of MgO in Al2O3 is small, ~300 ppm. This is due to the relatively large difference in ionic radius: 0.72A for Mg2+ and 0.53A for Al3+ . - Any fast migration of the grain boundary would have to incorporate Mg2+ ions into the Al2O3 lattice, with the resulting increase in internal energy, unless a NEW compound, spinel, forms. In Al2O3, oxygen diffusion along grain boundaries is faster than diffusion of aluminium on the surface or within the lattice. Therefore, the diffusion of aluminium, the slowest moving ion along the grain boundary, its fastest diffusion path, is the limiting factor in the densification of Al2O3. The solid solubility of MgO in Al2O3, although small (300-1400 ppm between 1600 and 1800C), creates interstitial Ali and thus increases the diffusivity of aluminium, increasing rate of densification relative to the rate of grain growth. Arguments on the role of MgO in Al2O3 sintering continue to the present time. Note that other grain-growth inhibitors can be added to Al2O3. For example, submicron ZrO2 particles (10-20 vol%) residing at the triple points of grain boundaries of alumina effectively inhibit grain growth according to the equation: r4 - r04 = a t, where: r = the average particle size after time t, r0 = the average initial particle size, t=0, and a = constant. Sintering of SiC As an another example consider sintering of silicon carbide. Pressureless sintered SiC requires B and C as additives, which affect the relative diffusion coefficients. Presence of silica impurities in SiC can enhance evaporation - condensation mechanisms through a volatilisation reaction: SiO2 (solid) + SiC (solid) SiO(gas) +CO (gas) Carbon removes SiO2 and Si from the surface of SiC according to the carbothermal reduction reaction: SiO2 + 3C SiC + 2CO ; C+Si SiC Thus, fewer defects are present at the surface and diffusion along the surface decreases. This slows down the coarsening mechanisms. Simultaneously, boron has been found to selectively segregate toward grain boundary regions, where its role is unclear, i.e. it is suspected that: - it lowers the grain boundary surface energy ss and thus increases the macroscopic driving force sv - ss - it introduces additional defects (silicon vacancies) and enhances grain boundary diffusion In general, the following characteristics determine sinterability of ceramic compacts: Powder: • fine; • equiaxed; • non-aggregated; • narrow size distribution powder compact • high green density; • no gross defects Powder compact: • high green density; • no gross defects Kinetics after compact formed and heated: • microstructure controlled by relative rates of coarsening and densification and by pore-grain boundary interactions • Pores: size; size distribution; location relative to grain boundary • Second phases: size; size distribution; location relative to grain boundary • Dopants/impurities: in solution; in second phases • Non-stoichiometry 5.2 Pressure Assisted Sintering 5.2.1. Pressure Assisted Sintering - Technology Pressure assisted sintering increases the contact pressure of particles and thus the driving force for sintering, compared to pressureless solid state sintering. by ~ one order of magnitude in hot pressing (HP) at 20-40 MPa pressure by ~ two orders of magnitude in hot isostatic pressing (HIP) at 200 - 300 MPa pressure The pressure applied to the external surface of a green compact is transmitted to the component’s interior through what is initially the much smaller effective surface of the contacting particles. The local contact pressure, or "effective pressure", drives localised particle deformation by yield and creep, and causes progressive densification. However, as densification proceeds, the contact area increases and the additional driving force for densification rapidly decreases. This results in apparent "geometrical hardening" of the hotpressed powdered compacts. Therefore, removal of the last few % of porosity in pressure assisted sintering is not much faster than in pressureless sintering. Furthermore, non-densifying mechanisms such as surface diffusion or vapour transport are NOT enhanced in pressure-sintering. The excess of driving force for sintering due to externally applied pressure is frequently used to decrease the processing temperature (usually by 100 to 500C) rather than to achieve rapid densification rates. Thus, fine-grained microstructures are obtained. Alternatively, materials which are difficult to sinter, such as composites or covalent solids, can be densified by pressure assisted techniques. New sintering mechanisms are added to solid-state sintering when pressure is applied. These are : - agglomerate fragmentation - particle rearrangement - plastic flow - diffusional creep The additional sinterability allows the compact to reach high density and to narrow the distribution of the residual flaw size (porosity). This usually translates to less scatter in component strength and a higher Weibull Modulus. 1. Hot Pressing This is the older technology of the two. In essence, it consists of a heated uniaxial press. It involves simple instrumentation AND poor shape capability. The pressure capability is limited by mould strength (20-40 MPa. 2. Hot Isostatic Pressing Here, GAS is the pressing medium, with pressure capabilities ~ 200 to 300 MPa. The pressure can only be transmitted to the interior of the component if the surface is SEALED using metal or glass cans, or predensification until closed (>92%TD) porosity is produced. In HIP-ping driving force is the same as in hot pressing , however additional diffusivity is provided from increase in stress at contact area. Sample must be jacketed , otherwise open pores will not be removed. The jacket materials include metal can (Pt. Ni, Fe, Ti, W ) or glass , must be viscous and non-penetrating the component. By HIP-ping we can densify many hard to densify materials. Jacketless variant is possible, where must sinter sample to the closed pore stage without pressure, and then apply pressure to fully densify the component. Location of pores after sintering is important: pores inside grains will remain, whereas pores on grain boundaries will be removed. 5.2.2. Pressure Assisted Sintering - Modeling Pressure-assisted densification kinetics are described as a combination of a density D - dependent function fi(D) and a function which depends on the parameters of the system, Ki. dD/dt = Ki fi(D) Ki and fi (D) vary, depending on the particular densification mechanism "i". For example, for densification by lattice diffusion at low densities (D<90%), Ki = 5.6DvMP/(RTr2) and fi(D) = (D - D0)-1 where: Dv = volume diffusion coefficient, M = molecular volume, P = pressure, R = gas constant, T = temperature, r = particle size, and D0 = initial density. Based on equations similar to the above, HIP diagrams can be designed,, indicating relative density as a function of the applied pressure, time, and temperature conditions. HIP diagrams show the regions of dominance of a particular densification mechanism (yield creep diffusion, with decreasing effective pressure). HIP diagrams allow the optimisation of HIP technology. Shape distortion in HIP can be a problem. It is caused by: - nonuniform mould filling (the final shape assumes equal density) - temperature gradients (initial thermal conductivity of compacts is low). Temperature gradients depend on the component size and its thermal conductivity. - The sintering rate’s sensitivity to temperature For the worst combination of above parameters (thick sections, low thermal conductivity, sintering rate changes rapidly with temperature), a densification front can develop (instead of uniform densification). Dramatic shape distortion results.