6- Sintering of Ceramics.

Ceramic and glass technology third stage 21
3- Drying
The moisture content expressed in eq.(1)
…………….(1)
18
Fig (18):
Ceramic and glass technology third stage 22
4- Firing
Once the green ceramic component has been cast by any of the methods described in the previous section, dried, and surface finished, it is generally subjected to a process known as firing. Firing involves heat treatment of the green ceramic in a high-temperature furnace, known as a kiln , to develop the desired microstructure and properties. Kiln design varies widely, depending upon the ceramic to be fired, but they are generally designed to operate in either an intermittent or continuous fashion. Heating can be supplied by heating elements such as silicon carbide (to 1150◦C), molybdenum disilicide (to 1700◦C), refractory metals such as molybdenum or tungsten (> 1700◦C ) , graphite (> 1700◦C in inert gases), plasma heating sources, microwave heating, or induction. Combustion heating is also commonly utilized, in which methane, usually in the form of natural gas, is combusted with air to produce temperatures in excess of 1700◦C. Several processes occur during firing, including further drying beyond that performed in the casting process, binder burn-out, and, most importantly, sintering.
5- Pre-sintering Processes.
Material changes that occur upon firing prior to sintering may include drying, the decomposition of organic additives, the vaporization of hydrolyzed water from the surfaces of particles, the pyrolysis of particulate organic materials, changes in the oxidation state of some of the transition metal and rare-earth ions, and the decomposition of carbonates and sulfates, termed calcination , that are introduced either as additives or as raw component constituents. The decomposition of organic additives, such as binders, called thermolysis , is an important step prior to densification on sintering.
Incomplete binder removal and uncontrolled thermolysis may reduce yields or impair performance of the product. Ideally, the green ceramic should survive thermolysis without deformation, distortion, the formation of cracks, or expanded pores. Binder burnout is dependent upon the composition of the binder and the composition and flow rate of the kiln atmosphere. It is also very dependent upon the microstructure of the organic, powder, and porosity phases and dynamic
Ceramic and glass technology third stage 23 changes in the microstructure as the binder is eliminated. Most cast ceramic components have sufficient pore channel sizes to allow the transport of vapors and gases from the binder burn-out zone to the product surface. The time for thermolysis is controlled by the diffusion length for vapor phase transport rather than by the characteristic dimension of the binder phase as shown below:
Decomposition and vaporization of the organics will cause an internal gas pressure that will depend on the gas evolution rate, gas permeability, and the compact size.
The permeability varies with the binder loading, and the pore structure varies with pore radius and volume fraction. Thus, the gas permeation rate is much lower in dense compacts of very fine particles. As an example of thermolysis, consider the burnout of polyvinyl alcohol from a barium titanate compact in air. Initially, there is loss of hydroxyl and hydrogen side groups, leaving a conjugated hydrocarbon:
The volume of the gaseous products is several hundred times larger than the volume of the compact. Other additives such as waxes and polyethylene glycols melt at relatively low temperatures and vaporize over a more narrow temperature range. For the thermolysis of polyethylene glycol (PEG) in air, decomposition occurs by the mechanism of chain scission and oxidative degradation. Thermolysis of an organic binder in an inert atmosphere such as nitrogen, as is used when firing silicon nitride and aluminum nitride, proceeds differently. Some initial oxidation of the binder may occur from adsorbed oxygen. Vaporization is more predominant and in general the thermolysis is endothermic and shifted to a higher temperature,
Some residual carbon may be expected in the absence of oxygen When firing nitride and carbide ceramics in an inert atmosphere, carbon is again the common
Ceramic and glass technology third stage 24 residue, which may serve as a sintering aid. Some ceramics, such as kaolin, contain waters of crystallization that are eliminated between 450 ◦ C and 700 ◦ C:
Al
2 o
3
. 2SiO
2
. 2H
2 o Al
2 o
3
. 2SiO
2
+ 2H
2 o ( g )
The gas pressure may produce a volume expansion, and the heating rate must be carefully controlled in bodies containing a high clay content. Other dehydration reactions producing gas are the dehydration of aluminum hydrates in the range
320–560 ◦ C and talc at 900–1000 ◦ C. Carbon dioxide is produced upon the decomposition of magnesium carbonate at 700 ◦ C and dolomite at 830–920 ◦ C.
Magnesium sulfate decomposes as low as 970 ◦ C, but the decomposition of calcium sulfate occurs in excess of 1050 ◦ C. Fine organic matter in clays is commonly oxidized between 200 ◦ C and 700 ◦ C, but coarse particulate carbon in the material may not be completely oxidized at 1000 ◦ C even when firing with excess air. Residual carbon discolors the microstructure and can lead to the production of carbon monoxide in the pores of the product. A chemical dopant such as a transition metal oxide in its higher oxidation state, such as Mn2O3, is sometimes added as an internal oxidizing agent, in which the following reaction occurs:
Carbon monoxide in the product is not inert and may change the stoichiometry and properties of transition metal oxide pigment or magnetic ferrites, such as Fe2O3.
6- Sintering of Ceramics.
6-1 Solid state sintering.
Sintering is a process in which a particulate material is consolidated during heat treatment. Consolidation implies that within the material, particles have joined together into an aggregate that has strength. Densification , or the increase in density through the accompanying reduction in interparticle voids, often accompanies sintering,. the criteria for sintering to occur:
A mechanism for material transport must be present.
Ceramic and glass technology third stage 25
A source of energy to activate and sustain this material transport must be present.
In Solid state Sintering involves materials transport by volume diffusion as shown in fig (19)
Three stages of solid-state sintering are recognized:
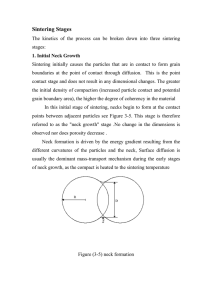
1- Initial sintering involves rearrangement of the powder particles and formation of a strong bond or neck at the contact points between particles (19.b). The relative density of the powder may increase from 0.5 to 0.6 due mostly to an increase in particle packing.
2- Intermediate sintering occurs as the necks grow, the number of pores decreases substantially, and shrinkage of the particle assembly occurs. Grain boundaries form, and some grains grow while others shrink. This stage continues while the pore channels are connected, called open porosity , but is considered over when the pores are isolated or in closed porosity. The majority of shrinkage occurs during intermediate sintering, and the relative density at the end of this stage is about 0.9.
3-Final-stage sintering occurs as the pores become closed and are slowly eliminated by diffusion of vacancies from the pores along the grain boundaries.
Little densification occurs in this stage, although grain sizes continue to grow.
Fig (19)Development of ceramic microstructure during sintering: (a) Loose powder particles; (b) initial stage; (c) intermediate stage; and (d) final stage.
Ceramic and glass technology third stage 26
These three stages, and the accompanying densification that occurs in each stage, can be observed for the sintering of two 0.8- (finer) and 1.3-μm (coarser), magnesia-doped alumina powders in Fig(20). For comparison, the densification process for hot pressing of the same powder is shown. Notice that most of the densification takes place in the intermediate stage.
Fig (20): Densification behavior of compacts of two alumina powders (0.8- and 1 .
3 –
μ m average particle size), with the sintering stages indicated for the coarser powder. A hot-pressed powder is shown for comparison.
The mass transport mechanisms that lead to densification include grain boundary diffusion, lattice diffusion, viscous flow, and plastic flow. Such mass transport processes as surface diffusion and evaporation take place during sintering, but do not contribute to densification. The shrinkage that often accompanies densification is controlled by different mechanisms in the three stages of sintering. In the initial stage of sintering, the linear shrinkage(equivalent to the sintering rate), ∆ L/L 0, is described by where Dv is the apparent diffusivity of the vacancies of volume Vv , d is the grain diameter, K is a geometry-dependent constant,
γ
s is the solid surface energy , t is time, k B is Boltzmann’s constant, T is absolute temperature, and m and n are constants depending on the mechanism of mass transport. The value of n is approximately 3, which implies that surface diffusion predominates, and m is in the
Ceramic and glass technology third stage 27 range of 0.3–0.5. The shrinkage is very temperature-dependent, as showm in fig(21), the diffusivity is also temperature dependent
Fig (21)
In the intermediate stage, can still apply, but the situation is complicated by grain growth (i.e., d is no longer constant) and a change in the pore geometry. More than one mass transport mechanism may be contributing to the changes in microstructure. In the final stages of sintering, densification is very dependent upon the association of pores with grain boundaries and the rate and mode of grain growth. Diffusion of atoms across the grain boundary causes the grain boundary to be displaced. The grain boundary mobility, Mb , can also be described by an
Arrhenius-type expression, so it is temperature-dependent. When Mb is roughly constant, it can be used to determine the rate of grain size growth with time, t : where n is the dimensionality parameter of with a value of 2–3, d
0
is the original mean grain size, dt is the mean grain size at time t, A is a constant that depends on geometry, and
γ
gb is the grain boundary interfacial energy. A log-normal grain size distribution is often observed during regular grain growth.
When grains grow so rapidly that pore are trapped with grains rather than moving along the grin boundaries until the pores are eliminated. This happens in pure
Al
2
O
3
were the complete microstructure consist of large grains with porosity traped within grains, this porosity is not easily removed during further sintering. This problem avoided by addition of 0.25 wt% MgO to Al
2
O
3
. MgO slowed the rate of motion of the grin boundaries until all porosity eliminated.
Ceramic and glass technology third stage 28
*** finer particle size powder can be sintered more rapidly and at lower temperature than coarser powder.
*** if particle packing is not uniform in green product , it will be very difficult to eliminate all the porosity during sintering.
*** particle shape can be important . too high concentration of elongated or flattened, producing a large or irregularly shaped pore that is difficult to remove during sintering
*** particle size distribution is also critical . particles that are all of one size are difficult to pack efficiently; they forms compact with large pores and high volume percentage of porosity.
Liquid phase sintering
For materials which are hard to sinter a process called liquid phase sintering is commonly used. Materials for which liquid phase sintering is common are Si
3
N
4
,
WC, SiC, and more. Liquid phase sintering is the process of adding an additive to the powder which will melt before the matrix phase. The process of liquid phase sintering has three stages:
– viscous flow and grain rearrangement: As the liquid melts capillary action will pull the liquid into pores and also cause grains to rearrange into a more favorable packing arrangement. The liquid dissolves the surface asperities and also dissolves the small particles.
– solution-reprecipitation: In areas where capillary pressures are high (particles are close together) atoms will preferentially go into solution and then precipitate in areas of lower chemical potential where particles are non close or in contact. The transfer of matter followed by reprecipitation in the low energy areas results in densification;
– development of the solid skeleton: the liquid phase is eliminated gradually by the formation of new crystals or solid solutions
For liquid phase sintering to be practical the major phase should be at least slightly soluble in the liquid phase and the additive should melt before any major sintering of the solid particulate network occurs, otherwise rearrangement of grains will not occur.