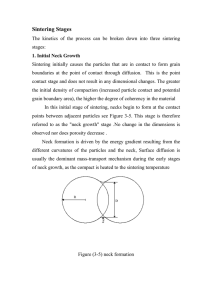
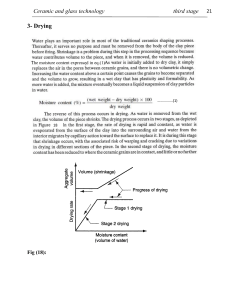
sintering
advertisement

Che5700 陶瓷粉末處理 Firing (Sintering) •To develop desired microstructure, hence desired product properties; to turn green body into final products • Usually last step of process, may sometime has an additional “post-sintering finish” •Three stages: (a) organic burnout, elimination of gaseous products from decomposition and oxidation; (b) sintering; and (c) cooling, may include thermal and chemical annealing; 1 Purpose of sintering: develop necessary strength (through consolidation of particles; or densification) particles join together via solid state diffusion and/or reaction to form compound (sometimes with the help of small amount of liquid phase) elimination of pores shrinkage of product Sometimes, the end product can be very “porous” (e.g. membrane, filter, etc) 2 Equipments furnaces, tunnel kilns (picture taken from Google search) many different designs: provide enough temperature, time and atmosphere for sintering 3 Presintering Processes Thermolysis: organic burnout; important step before sintering 4 Thermolysis Behaviour May be influenced by: binder concentration, product size, product placement configuration, heating rate, furnace atmosphere; Time required: probably by diffusion length of vapor phase, may take hours, or days 5 Other Considerations Endothermic or exothermic reactions may alter the internal temperature and rate of reactions Gas permeation rate slow in compact from fine particles; amount of gaseous product; ex: PVA, initial endothermic, later exothermic reactions 6 Thermal Analysis TGA, DTG, DTA, DSC (differential scanning calorimetry), TMA (thermal mechanical analysis) Many variations of TMA: measure volume changes (e.g. creep behaviour), etc. 7 Quite different behavior between different cases 8 More Examples 9 Effect of Atmosphere Plasticizer (low MW) helpful to generate interconnecting pores for later decomposition of binders for binders (high MW): degradation & oxidation Ash from burnout: may not be overlooked 10 Decomposition of Inorganic Compounds Dehydration reactions (water of crystallization) : clay 450 – 700oC; talc 900-1000oC Carbonates: usually 700 – 920oC Sulfates: higher temperature is often required some organic matter may produce “carbon”, cause black color in final product C + deficiency in oxygen CO, may have effect on oxidation state of final product, e.g. Mn2O3 & MnO or Fe3O4 & Fe2O3 & FeO 11 Solid State Sintering Pure sintering, sintering with reactions and dissolving, sintering in the presence of glass particles, etc. Density change to indicate the degree of sintering 12 Driving force of sintering: Gt = Gv + Gb + Gs (t: total; v: volume; b: boundary; s: surface of grain) Simply put: elimination of pores, elimination of free surface area associated with these pores Densification rate & final degree of densification: hot press > sintering with fine particle > sintering with coarse particle 13 14 surface diffusion: produce surface smoothing, particle joining, pore rounding (no shrinkage) Viscous flow & plastic deformation: major effect on volume change Pores: source of vacancy; grain boundary: vacancy sink 15 Homogeneous vs inhomogeneous systems 16 Ball mill to reduce aggregate size helps sintering 17 Grains with more than 6 sides, have concave boundary log-normal grain size distribution; uniformity is key Arrows indicate direction of movement Large becomes larger; to minimize grain boundary grains with less than 6 sides, have convex boundary. Tend to shrink 18 Regular grain growth vs exaggerated (or discontinuous) grain growth average grain size d = 1.56L with L = read from mean intercept length between boundaries 19 Dopant accumulate in the boundary, reduce surface tension grain growth inhibitor: segregated at grain boundary, help to get high density product 20 Many models proposed for grain growth, or pore mobility: e.g. Mp = K Ds/(T rp^4) [Ds suface diffusion coeff.; rp pore radius] small pores moves fast vacancy diffuse from small pores to large pores an example of Ostwald ripening densification faster: if vacancy diffusivity > surface diffusivity (grain coarsening); [fast heating rate is useful] 21 Rapid heating rate : densification with small grain size; Surface diffusion dominate at low temperature favors grain coarsening 22 The main results are: (i) both n-TiO2 and ZrO2 undergo densification at temperature much lower than they do in more conventional sized powders; due to small size (ii) they densify without significant grain growth until the density reaches ,- 90% bulk density and the porosity becomes closed; (iii) at densities above 90%, grain growth can be rapid; abnormal grain growth, however, was never observed; 23 source: Nanostructured Materials, 1 (1992) 173-178 24 (iv) grain growth can be controlled by pressure-assisted sintering or by Y doping in n-TiO2, although complete densification appears to require some grain growth (v) Vickers hardness in dense nanophase ceramics are as high as in single crystal TiO2, but it decreases with increasing grain size on annealing. 25 Impurity doping: effective to limit grain growth Vickers hardness ~ d^-1/2 26 Atmosphere important: gas trapped in closed pore will limit its shrinkage unless gas is soluble in the ceramics SO2, Cl2 may come from impurity in raw particles; oxygen may be important in determining the stoichiometry of product (e.g. ZnO, PbO. Ferrites etc.); lattice vacancy & oxidation state in nitrogen atmosphere: reaction bonded Si3N4 27 Compact pressed at high pressure narrow & smaller pore size higher density In a uniform compact: uniform interstices smaller than grain size shrink at a fast rate; coarse micropore: usually grow very large macro-pore: remain the same during sintering 28 Sintering in the presence of small amount of wetting liquid Usually a glassy phase wetting grain; better densification at lower temperature This glassy phase is also beneficial to adherence to substrate or glaze 29 Less than 1% glassy phase is sufficient if distributed uniformly; High diffusivity in the liquid phase increase mass transport and shrinkage Liquid penetrate between grains, inhibiting exaggerated grain growth 30 Sintering of whiteware bodies (vitrification) Densification occurs simultaneously with reaction and dissolving of raw materials producing new glassy and crystalline phases Fine clay coat quartz and feldspar (KAlSi3O8 – NaAlSi3O8 – CaAl2Si2O8) quartz dissolving slowly into feldspar liquid above 1250C, producing mullite; whole process very dependent on particle size & impurities high green density & control of heating program are important 31 Around 560C, dehydration from clay; Shrinkage at 950C to formation of mullite (3Al2O3 2 SiO2) from metakolin 1160C change in vitrification 32 Sintering of glaze and glassy thick film Glaze commonly < 1 mm in thickness Spreading of the vitreous phase: gravity & surface tension & viscosity of the glaze (both dependent on temperature & composition) lead frit is often added; yet lead oxide is volatile (air pollution problem) Bubbles diffuse out of glaze Some glaze penetrate & react with substrate body; develop strong bonding; pigment may settle in glaze one-fire, two-fire processes 33 34 One example of manganese zinc ferrite: presintering, sintering, annealing, cooling; different atmosphere; Sintering with high oxygen pressure to prevent loss of ZnO; during cooling, reduced O2 pressure to get ferrous iron for single phase product; 35 Summary Ceramics are produced in a variety of size, shape, composition, & production rate; General procedure often contains presintering, sintering, annealing and cooling stages; heating rate, time & atmosphere can be changed elimination of pores is desired; closed pores will limit final density; grain growth also occur during sintering final microstructure final property 36