quiz3-0405ans
advertisement

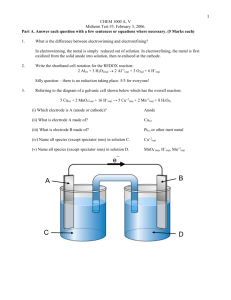
1 Name:__________________________________________________ Student Number:__________________ CHEM 1000 A, V Midterm Test #3, February 4, 2005 Calculator allowed. This test should have six pages. A periodic table and a table of reduction potentials are on the last page. Part A. Answer each question with a few sentences or equations where necessary. (5 Marks each) 1. What prevents primary Galvanic cells from being recharged? The products of the reaction do not stick to the electrodes and are therefore not in electrical contact with the charging circuit. 2. Write the shorthand cell notation for the REDOX reaction: Cr2O7-2(aq) + 6 Br(aq) + 14 H+(aq) 2 Cr+3(aq) + 3 Br2(l) + 7 H2O(l) Pt(s) Br-(aq) Br2(l) Cr2O7-2(aq), Cr+3(aq), H+(aq) Pt(s) 3. What is overpotential? Overpotential is the potential over and above the standard cell potential that must be applied to make an electrolytic reaction proceed. 4. Arrange Cd(s), Fe(s) and Zn(s) in increasing order of reducing strength and explain your reasoning. Referring to the reduction potential table, Cd is above Fe, which is above Zn. Thus, Zn must be the most easily oxidized of the three metals, and must therefore be the strongest reducing agent. 5. Name the strongest intermolecular force between two molecules of SO2. SO2 is a bent molecule, and is therefore polar. Two SO2 molecules will therefore interact via dipole-dipole forces. 6. Why does the freezing point of water decrease as the pressure is increased? Increasing the pressure tends to make the system more dense. Liquid water is more dense than ice. Increasing the pressure therefore moves the equilibrium towards the liquid, i.e. the solid melts. Part B. Answer any three questions. If you answer all four, the best three will be used to calculate your mark. (20 marks each) B1. Referring to the phase diagram for carbon below, 106 A C E 104 p, atm 102 F B D 1 0 6000 T, K (a) What phases are present at the following points? 2 [1] [1] [1] [1] [3] [3] A: Diamond B: Graphite C: Liquid D: Gas E: Diamond, graphite and liquid F: Graphite, gas and liquid [2] (b) What are points E and F called? Triple points [2] (c) Does the melting point of carbon increase or decrease as the pressure is increased? How can you tell? The melting point curve has a positive slope, thus the melting point increases as the pressure increases. [4] (d) What happens as a sample of carbon at 100 atm pressure is heated from 1000 to 6000 K? At 100 atm and 1000 K, the carbon is in the form of graphite. At approximately 4000 K, gaseous carbon appears and is in equilibrium with the graphite. Above this temperature, the graphite disappears and there is only gaseous carbon. [2] (e) Graphite is the standard state of carbon. Why do diamonds not appear to spontaneously change to graphite? Diamonds are constantly changing to graphite, but it is too slow to be noticed. B2. The reactions in a lead-acid battery are: Pb(s) + HSO4 (aq) PbSO4(s) + H+(aq) + 2e PbO2(s) + 3 H+(aq) + HSO4 (aq) + 2e PbSO4(s) + 2 H2O(l) [2] (a)Write the overall reaction. Pb(s) + PbO2(s) + 2 HSO4 (aq) + 2 H+(aq) 2 PbSO4(s) + 2 H2O(l) [2] (b)Calculate the standard cell potential (V). Eocell = Eocathode + Eoanode = 1.63 V + 0.30 V = 1.93 V [2] (c) Calculate the standard free energy change for the reaction (kJ) Go = -nFEo = 2 mol e (96487 C (mol e)1(1.93 J C1) = 372000 J = 327 kJ Eo = 0.30 V Eo = 1.63 V 3 [14] -1 o (d) Calculate [H2SO4(aq)] (mol L ) if the cell potential is 1.89 V at -10 C. RT ln(Q) nF nF o or, ln(Q) (E E) RT 2 (96487 C mol ) (1.93 1.89) J C1 1 1 8.314 J K mol (263K) E Eo 3.53 thus, Q e3.53 34.1 For this reaction, Q 1 [HSO4 (aq) ]2 [H (aq) ]2 thus, [HSO4 (aq) ]2 [H (aq) ]2 1/ 34.1 0.0239 thus, [HSO4 (aq) ][H (aq) ] 0.0239 0.155 In solution, H2SO4 H+(aq) + HSO4-(aq). Thus, [H+(aq)] = [HSO4-(aq)] = (0.155)1/2 = 0.393 M In other words, [H2SO4(aq)] = 0.393 M 4 [18] B3. (a) A surface is to be electroplated with calcium (Ca) metal by electrolyzing molten CaCl2. If a surface is made the cathode in an electrolytic cell, how long (in minutes) will it take to deposit a layer of Ca(s) 0.015 mm thick on an object having a surface area of 80.0 cm2, using a current of 0.12 A? The density of calcium metal is 1.55 g/cm3. First, we find the amount of Ca to be deposited: 0.0015 cm x 80.0 cm2 = 0.12 cm3 0.12 cm3 x 1.55 g cm3 = 0.186 g Ca 0.186 g Ca / 40.08 g mol1 = 0.00464 mol Ca The reduction reaction is Ca+2 + 2 e Ca(s). Thus, two moles of electrons are required per mole of calcium deposited. Thus, moles electrons = 2(0.00464) = 0.00928 moles electrons (= n) A current of 0.12 A is 0.12 C/s it nF t nF 0.00928 mol e (96487 C (mol e ) 1 ) i 0.12 Cs 1 7463s 124 min [2] (b) Why can calcium not be reduced from an aqueous solution of CaCl2? Water will be preferentially reduced, since it has a higher reduction potential than Ca +2(aq). [20] B4. The vapor pressure of cyclohexane at 25.0oC is 0.130 atm. Its enthalpy of vaporization is 31.8 kJ mol-1. Calculate the normal boiling point of cyclohexane (oC). In this case, let T2 = 25.0oC = 298 K, then p2 = 0.130 atm. We need to solve for the normal boiling point T1, which will occur at 1.00 atm by definition. Thus, p1 = 1.00 atm. p H vap ln 2 R p1 1 1 T2 T1 1 1 p R ln 2 T1 T2 H vap p1 p 1 1 R ln 2 T1 H vap p1 T2 R p 1 T1 ln 2 H vap p1 T2 1 8.314 J K 1mol 1 0.130 1 ln 1 1.00 298 31,800 J mol 354 K 81o C 1