Functional Endoscopic Sinus Surgery CG-SURG-24
advertisement

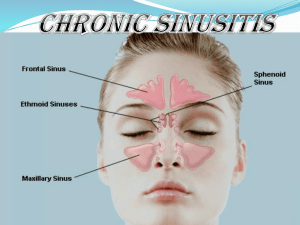
REVIEW REQUEST FOR Functional Endoscopic Sinus Surgery (FESS) Provider Data Collection Tool Based on Clinical Guideline CG-SURG-24 Policy Last Review Date: 08/06/2015 Policy Effective Date: 10/06/2015 Provider Tool Effective Date: 12/12/2015 Individual’s Name: Date of Birth: Insurance Identification Number: Individual’s Phone Number: Ordering Provider Name & Specialty: Provider ID Number: Office Address: Office Phone Number: Office Fax Number: Rendering Provider Name & Specialty: Provider ID Number: Office Address: Office Phone Number: Office Fax Number: Facility Name: Facility ID Number: Facility Address: Date/Date Range of Service: Service Requested (CPT if known): Place of Service: Outpatient Home Inpatient Other: Diagnosis Code(s) (if known): This clinical guideline based data collection tool is for a medical necessity review request for functional endoscopic sinus surgery (FESS), an endoscopic surgical procedure used to treat various conditions of the nasal sinuses including but not limited to chronic sinusitis. Please check all that apply to the individual: □ The request is for functional endoscopic sinus surgery (FESS) for the treatment of sinusitis, polyposis, or sinus tumor (If checked , mark all that apply to the individual) □ Individual has uncomplicated sinusitis (for example, sinusitis confined to the paranasal sinuses without adjacent involvement of neurologic, soft tissue, or bony structures) (If checked, mark all of the following that apply to the individual) □ Individual has had 4 or more documented episodes of acute rhinosinusitis (for example, less than 4 weeks duration) in 1 year □ Individual has chronic sinusitis (for example, greater than 12 weeks duration) that interferes with lifestyle □ Maximal medical therapy has been attempted (If checked, mark all of the following that apply to the individual) □ Antibiotic therapy for at least 4 consecutive weeks □ Trial of inhaled steroids □ Nasal lavage □ Allergy testing (if symptoms are consistent with allergic rhinitis and have not responded to appropriate environmental controls and pharmacotherapy [antihistamines, intranasal corticosteroids, leukotriene antagonists, etc.]) REVIEW REQUEST FOR Functional Endoscopic Sinus Surgery (FESS) Provider Data Collection Tool Based on Clinical Guideline CG-SURG-24 Policy Last Review Date: 08/06/2015 Policy Effective Date: 10/06/2015 Provider Tool Effective Date: 12/12/2015 □ Individual has abnormal findings from a diagnostic work-up documented in the medical record (If checked, mark any of the following that apply to the individual) □ CT findings suggestive of obstruction or infection (for example, air fluid levels, air bubbles, significant mucosal thickening, pansinusitis, or diffuse opacification) □ Nasal endoscopy findings suggestive of significant disease □ Physical exam findings suggestive of chronic/recurrent disease (for example, mucopurulence, erythema, edema, inflammation) □ Individual has a suspected tumor seen on imaging, physical examination, or endoscopy □ Individual has suppurative (pus forming) complications (If checked, mark all of the following that apply to the individual) □ Subperiosteal abscess □ Brain abscess □ Other complication not identified above: _______________ □ Individual has chronic polyposis with symptoms unresponsive to medical therapy □ Individual has allergic fungal sinusitis as indicated by (If checked, mark all of the following that apply to the individual) □ Nasal polyposis □ Positive CT findings □ Eosinophilic mucus □ Individual has a mucocele causing chronic sinusitis □ Individual has recurrent sinusitis that triggers or aggravates pulmonary disease (such as asthma or cystic fibrosis □ Individual has fungal mycetoma □ Individual has failed other sinus surgery □ Individual has cerebrospinal fluid rhinorrhea □ Individual has an encephalocele □ Individual has posterior epistaxis □ Individual has persistent facial pain after other causes are ruled out □ Individual has cavernous sinus thrombosis caused by chronic sinusitis □ Request is for nasal or sinus cavity debridement following FESS □ Debridement up to two times during the first 30 days postoperatively □ Individual has postoperative loss of vision or double vision □ Individual has evidence of cerebrospinal fluid leak such as rhinorrhea □ Individual has physical obstruction of the sinus opening as indicated by: (If checked, mark any of the following that apply to the individual) □ Nasal polyps unresponsive to oral or nasal steroids □ Documented presence of papilloma, carcinoma or other neoplasm □ Allergic fungal sinusitis REVIEW REQUEST FOR Functional Endoscopic Sinus Surgery (FESS) Provider Data Collection Tool Based on Clinical Guideline CG-SURG-24 Policy Last Review Date: 08/06/2015 Policy Effective Date: 10/06/2015 Provider Tool Effective Date: 12/12/2015 This request is being submitted: Pre-Claim Post–Claim. If checked, please attach the claim or indicate the claim number I attest the information provided is true and accurate to the best of my knowledge. I understand that the health plan or its designees may perform a routine audit and request the medical documentation to verify the accuracy of the information reported on this form. Name and Title of Provider or Provider Representative Completing Form and Attestation (Please Print)* Date *The attestation fields must be completed by a provider or provider representative in order for the tool to be accepted. Anthem Blue Cross is the trade name of Blue Cross of California. Anthem Blue Cross and Anthem Blue Cross Life and Health Insurance Company are independent licensees of the Blue Cross Association. ANTHEM is a registered trademark of Anthem Insurance Companies, Inc. The Blue Cross name and symbol are registered marks of the Blue Cross Association.