Manual - Department of Chemistry
advertisement

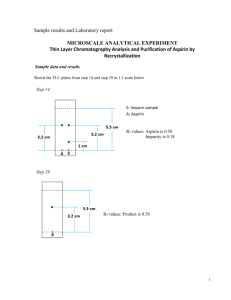
EDB workshop The Hong Kong University of Science and Technology Department of Chemistry MICROSCALE ANALYTICAL EXPERIMENT Thin Layer Chromatography Analysis and Purification of Aspirin by Recrystallization Dr TSUI, W. M. Basic Safety & Waste Disposal Procedures Personal Safety 1. Safety glasses must be worn at all times in the labs. Contact lenses should not be worn in the lab because chemicals and particulates can get caught behind them, causing severe eye damage. 2. Lab gown must be worn in the labs where an unexpected chemical spill may expose you to the risk of injury. The following clothing is not permitted in the labs unless covered by protective clothing: Open-toed shoes, sandals or other uncovered footwear; clothes that expose above the ankles; Eating, chewing gum, and drinking in the lab. Untied long hair, dangling jewelry, loose clothing, and anything else that may get caught in equipment, or dipped in chemicals. 3. Never work alone or unsupervised in the labs. Work only during the scheduled laboratory periods and perform only authorized experiments. Wash your hands, arms, and then face, with soap and water as soon as possible after leaving the lab. If you are uncertain about any safety aspect of an experiment, please ask your TA. Lab Safety Make sure you know the exact locations of the safety features of the lab; e.g., eyewash fountains, safety showers, chemical spill kits, fire extinguishers, fire alarms, fire blankets. Whenever possible, do not deal with incidents on your own. Your TA, the lab instructor and the technician are all trained to respond to the sort of incidents that may occur in this lab; e.g. chemical spills, cuts, burns, fires, medical emergencies, etc. Keep your work area clean and organized to reduce the possibility of accidents. Know what you are doing and don't be careless. Avoid unnecessary exposure to chemicals. Never pipette by mouth. Never taste or inhale a chemical on purpose. Wear gloves when directly working with hazardous chemicals. Use hoods when appropriate. 1 EDB workshop Take appropriate precautions. Keep flammables away from hot plates and open flames. Wear gloves when using toxic, carcinogenic, or other hazardous chemicals. Take care with corrosive acids and bases. Always pour concentrated acid slowly into water (never water into acid). Read the Safety Issues section at the beginning of each experiment. Be informed. Material Safety Data Sheets (MSDS) summarize known hazards associated with every chemical are available from the lab. Chemicals & equipment may not be removed from the lab without permission from the Lab Instructor. Disposal of Chemical Waste It is very important to properly dispose the chemical waste you generate. Follow these guidelines and dispose of your waste properly, to avoid adding to the contamination of our environment. • Generate as little waste as possible. It is expensive to have hazardous waste removed and disposed. Don't prepare more of a chemical than you expect to use. • Never return unused portions of chemicals to the reagent bottle. At the end of your experiment, unused reagent must be disposed of as waste, so don't pour out more than you need. • Don't discard chemicals down the sink or in the wastebasket, unless you are explicitly told that it's okay to do so. Most of your chemicals will pose a threat to the environment if disposed improperly. • Place chemical waste only in the appropriate container. Often, more than one waste container is provided to separate certain chemicals for safety or easier disposal. Pay attention to the Waste Disposal information for each experiment in this lab manual, and use the waste containers indicated. If you cannot find a waste bottle labeled with your particular chemical, ask your TA where to dispose it. • Fill in the appropriate waste inventory sheet. There is separate inventory sheet for each waste container. Use it to record the chemical(s), concentrations and volume you dispose. • Do not over-fill a waste container. Tell the technician the bottle is getting full and they will replace it. • Use the clearly marked GLASS containers to dispose of broken glass and Pasteur pipettes. Do not place broken glass in the sink or wastebasket, to avoid serious injury to an unsuspecting person. • Use the clearly marked WASTE SOLIDS wide-mouth bottles to dispose of waste solids. Waste solids include solid chemicals, filter paper, and weighing paper. • If you realize that you disposed of a chemical in the wrong container, use the waste inventory list provided by the waste container to let us know. • If you have waste whose identity you can’t recall, you can often test your waste, e.g., with litmus paper, to deduce its identity. Do not add unidentified waste to the waste bottles. You will force us to categorize the entire waste container as “unknown” which becomes extremely expensive to identify and dispose of. 2 EDB workshop DEPARTMENT OF CHEMISTRY Hazard Identification Sheet Name: ________________________ Experiment Title: MICROSCALE ANALYTICAL EXPERIMENT – Thin Layer Chromatography Analysis and Purification of Aspirin by Recrystallization Date of Experiment: ________________________________ I have read and understand the instructions stated in ‘Basic Safety & Waste Disposal Procedures’ concerning the proper use of the chemicals. I shall work in compliance with the aforementioned instructions made by the ‘Basic Safety & Waste Disposal Procedures’. SECTION I: General Hazards Identified for the Experiment I shall handle hazardous inorganic chemicals during the experiment. I shall follow the instructions specified in ‘Basic Safety & Waste Disposal Procedures’. I shall handle organic solvents and chemicals during the experiment. I shall follow the instructions specified in ‘Basic Safety & Waste Disposal Procedures’. I shall dispose hazardous waste chemicals during the experiment. I shall follow the instructions specified in ‘Basic Safety & Waste Disposal Procedures’. [please tick where it is appropriate] 3 EDB workshop SECTION II: Specific Chemical Hazards For classification of hazards, please check the Material Safety Data Sheet (MSDS) established by The Sigma-Aldrich Company at: http://www.sigmaaldrich.com/chemistry.html Hazard Flammable Corrosive Toxic Irritant Harmful Oxidizing Explosive Others Chemical Aspirin Acetic acid Dichloromethane Ethyl acetate [please tick where it is appropriate] * Others: Carcinogenic, Allergic, Lachrymatory, Narcotic, Teratogenic, Reproductive hazard, Central nervous system depression, Dangerous to the environment, Asphyxiant, Poison SECTION III: Declaration This form to be completed by participant: I am well aware of the hazard(s) of the experiment(s) to be carried out in the Chemistry Laboratory. Name: Signature: Date: (in block letters) 4 EDB workshop The Hong Kong University of Science and Technology Department of Chemistry MICROSCALE ANALYTICAL EXPERIMENT Layer Chromatography Analysis and Purification of Aspirin by Recrystallization Dr TSUI, W. M. Chemical hazard notes Dichloromethane is harmful, irritant and carcinogenic; it is an asphyxiant, can cause central nervous system depression and reproductive hazard. Disposal of wastes Organic reagents and solvent must be disposed to appropriate waste solvent bottle. Introduction Chromatography is the separation of two or more compounds or ions by the distribution between two phases, one which is moving and the other which is stationary. These two phases can be solid-liquid, liquid-liquid or gas-liquid. Although there are many different variations of chromatography, the principles are essentially the same. For example, in analyzing an ink mixture using paper chromatography, the cellulose paper was the stationary or solid phase and the water mixture was the mobile or liquid phase as shown in the following diagram. 5 EDB workshop TLC Theory All forms of chromatography involve a dynamic and rapid equilibrium of molecules between the two phases. As shown in the Figure, there are: Free - completely dissolved in the liquid mobile phase Adsorbed - stuck on the surface of the solid stationary phase. Molecules A and B are continuously moving back and forth between the free and adsorbed states. Eventually, equilibrium is established between the Free and Adsorbed forms. Different molecules partition differently between the Free and Adsorbed forms, i.e. the equilibria between these two states is not the same. In the figure, molecule A is weakly adsorbed, its equilibrium lies in the direction of the Free form and there is a higher concentration in the mobile phase. Molecule B, on the other hand, is strongly adsorbed, its equilibrium lies in the direction of the Adsorbed form, and has a higher concentration on the stationary phase. Since the A molecules spend more time in the mobile phase, they will be carried through the stationary phase faster and move farther in a given amount of time. Since B is adsorbed to the stationary phase more than A, B molecules spend less time in the mobile phase and therefore move through the stationary phase particles more slowly. As a result, compound A and B are separated on the stationary phase. Rf value The Rf value is the “retardation factor” or the “ratio-to-front” value expressed as a decimal fraction. The Rf value can be calculated as: This number can be calculated for each spot observed on a TLC plate. Essentially it describes the distance traveled by the individual components. If two spots travel the same distance or have the same Rf value then it might be concluded that the two components are the same molecule. 6 EDB workshop Recrystallization Theory Most of the time, an organic compound that has been synthesized or otherwise obtained will also contain undesirable impurities. Recrystallization is one of the common techniques especially for “cleaning up” the impure samples in chemical industry. Recrystallization involves dissolving the impure solid in the minimum volume of a hot solvent and filtering to remove insoluble impurities (if any). The resulting hot and supersaturated solution of the compound, together with any soluble impurities is set aside to cool slowly, whereupon crystals of pure compound will separate from solution. The reason for the obtained crystals being pure is that the recrystallization process is an equilibrium, i.e. molecules in solution are in equilibrium with those in the crystal lattice. Since a crystal lattice is highly ordered, other different molecules, such as impurities, will be excluded from the lattice and will return to the solution. For a recrystallization to be successful, the solution must be allowed to cool slowly, so that the crystals are formed slowly, and the equilibrium process which excludes the impurities is allowed to operate. If a solution is cooled rapidly, impure molecules will be trapped or included in the rapidly growing crystal lattice. This rapid formation of solid material from solution is precipitation, and is not the same as recrystallization. 7 EDB workshop One of your tasks today is to analyze an impure sample of aspirin by TLC. After identifying the Rf value of both the impurity and pure aspirin, you have to purify the impure sample by recrystallization. TLC technique will be used again to check the final purity of your recrystallized product. Once you have purified your aspirin, you will also determine its melting point and percentage recovery. The purified aspirin will be further characterized by Infrared Spectroscopy. Procedure A: Thin layer chromatography 1. Draw a light pencil line across the plate at about 0.5 cm from the bottom of a TLC plate. 2. Mark two vertical dashes with equal spacing on this line and label the lanes as Sample (S) and Aspirin (A). 3. With the spotting capillary tubes, practice spotting on a paper towel (tissue) using a pure solvent such as dichloromethane. 4. With no more than 1 mg of impure aspirin, taken out from Sample (S), in an empty vial, dissolve it with 10 drops of dichloromethane so as to dissolve all the powder. Repeat this with Aspirin (A) in another empty vial. 5. After filling the capillary by dipping it in the impure aspirin sample solution, spot the plate on the S origin. Make each spot as small as possible, preferably no larger than 2 mm in diameter. 6. After the solvent evaporates, you can apply more material on the same spot by, again, quickly touching the surface at the same place. 8 EDB workshop 7. Repeat steps from 5 to 6 with authentic sample of aspirin solution and spot on the A origin of the plate. 8. Examine the plate under UV lamp to check if enough amount of the compound has been applied. You can observe a visible dark purple dot if enough; if not visible, spot more. 9. Develop your spotted TLC plate with a TLC developing chamber found on your bench. Pour the developing solvent into the bottle to a depth of about 0.25 cm. 10. Using forceps, carefully place the TLC plate into the bottles so that it is leaning against the wall of the bottle. Be sure that the spots are not below the solvent level or they will be washed away into the solvent. 11. Put on the lid to avoid excessive evaporation of developing solvent. After the solvent has risen to about 0.5 cm below the top of the plate, remove the plate with forceps and immediately mark the solvent front with a pencil. Allow the solvent to completely evaporate. 12. Examine the plate under UV lamp, you can see the components appear as dark spots against a bright green background. Outline the spots with a pencil. 13. Alternatively, the spots can be visualized by staining the plate with bromocresol green reagent, which can be found in fumehood. This can be done by putting the TLC plate into the bottle containing and stain reagent and then heat the plate gently with a hot gun. 14. Sketch the TLC results in your datasheet in 1:1 scale and calculate the Rf value for each compound. B: Recrystallization of Impure Aspirin 15. Weigh about 0.200g of impure aspirin sample with a 3-decimal-place balance. Add the sample into an Erlenmeyer flask. Place a wooden boiling stick in the flask to prevent bumping. 16. Slowly add deionized water into the flask and heat the solution to boil. 17. If there is still solid remaining, slowly add more water to the solution until all solid dissolves. 9 EDB workshop 18. Remove the Erlenmeyer flask from the heater and let it cool slowly to room temperature on bench. 19. Then, cool the Erlenmeyer flask in ice bath for 10 minutes. 20. At the same time, cool a capped test tube of DI water (about 15mL) in the ice bath. 21. Assemble the setup for suction filtration by placing an Hirsch funnel on a filter flask with a connector. See the figure on the right. 22. Place a filter paper into the funnel, turn on the water aspirator and connect the rubber hose to the filter flask as shown in the figure. 23. Pour the ice-cold crystalline mixture onto the Hirsch funnel. 24. Remove the rubber hose and rinse the crystal with few drops of ice-cold DI water. 25. Apply the vacuum again by connecting the rubber hose to the filter flask. 26. Repeat this washing process as necessary. 27. Keep the suction on and dry the crystals for 5 minutes. 28. Preweigh an empty vial. Collect all recrystallized product into the vial and weigh it again. 29. Obtain a few mg of recrystallized product from step 28 and repeat steps 1 – 14 to run a TLC experiment for the purified aspirin. 30. Compare the purity of your compound by juxtaposing your TLC result in step 14 and step 29. 31. Determine the melting point and obtain an IR spectrum of your purified aspirin. 10 EDB workshop Data Sketch the TLC plates from step 14 and step 29 in 1:1 scale below Rf values = Weight of empty vial = ____________ Weight of vial and recrystallized product = ____________ Weight of recrystallized product = ____________ Melting point of purified aspirin = _________________ Questions 1. Calculate the percentage recovery of your purified aspirin. 2. Compare the TLC result obtain from step 14 and step 29, comment on the purity of your recrystallized aspirin. 3. Interpret the IR spectrum of your purified aspirin. 11