Notes
advertisement

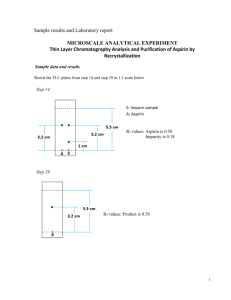
Notes for Teacher MICROSCALE ANALYTICAL EXPERIMENT Thin Layer Chromatography Analysis and Purification of Aspirin by Recrystallization A. Materials Chemical Acetic acid Aspirin Caffeine (as impurity in sample) Dichloromethane Deionized water Ethyl acetate Glassware Beaker (plastic) Boiling stick 50-mL Conical flask Cotton glove Developing bottle (for TLC) Filter adapter Forceps Hirsh funnel Hot plate stirrer 10-mL Measuring cylinder Spatula 25-mL Suction flask Stand and clamp Vial B. Preparation of materials Caffeine is used as the impurity in this experiment, and the impure aspirin sample (S) for TLC analysis and recrystallization is prepared by mixing aspirin and caffeine (2% w/w) in their powder form. Development solvent for TLC analysis is prepared by mixing 10 mL of ethyl acetate and 10 drops of acetic acid. 1 Bromocresol green stain reagent is prepared by dissolving 0.04 g of bromocresol green in 100 mL of absolute ethanol, then add 0.1 M solution of aqueous NaOH dropwise until a blue color just appears in solution. Ideally, this stain is to be stored in a 100-mL wide mouth jar. The lifetime of such a solution typically depends upon solvent evaporation. Thus, it would be advantageous to tightly seal such jars in-between uses. The melting point of the compound in this experiment is determined by Electrothermal IA9100 Melting point apparatus. The IR spectrum in this experiment is recorded by PerkinElmer Fr-IR Spectrum RXI Express system. C. Suggested time for the experiment Part A: Thin layer chromatography: 15 minutes Part B: Recrystallization of impure aspirin: 55 minutes Melting point determination: 15 minutes IR spectrum recording & sample preparation: 20 minutes Total: 105 minutes D. Instruction for students Experiment focus: - Theory and techniques in TLC analysis - Theory and techniques in recrystallization, including making a supersaturated solution for recrystallization, completely dissolve the impure sample before cooling, slow cooling of the hot supersaturated solution for forming crystals of bigger size and higher purity. - TLC as a rapid, effective and simple method in analyzing the purity of a compound. - Recrystallization as powerful purification method for solid sample - Melting point as another method for characterization and determination of purity - IR spectrum interpretation. E. Student's common errors and possible mistakes and Suggestion to avoid the errors and mistakes 1. Error: Too much developing solvent is added to the developing bottle, so some of the samples spotted on the TLC plate being dissolved in the solvent. Suggestion: Add developing solvent slowly to the bottle to control the amount added. 2. Error: Students used to hold TLC plates with fingers, this can cause contamination. 3. Error: Not marking the spots with pencil immediately after developing may result in disappearance of the spots. 4. Error: Recrystallization: Adding too much water for dissolution of impure sample cause no crystal form after cooling down. 2 Suggestion: 5 mL of water is enough to dissolve all the solid, students are advised to add a small amount (~ 3 – 4 mL) of water first, then heat the solution to boil, add more water (1 – 2 mL) if there is still solid remaining. Add more water before boiling can cause the above error easily. If it is the case, one can concentrate the solution by evaporation in a warm water bath. The solution should be supersaturated for crystals formation. 5. Error: The flask in step 18 is put to the ice bath before completely cooling down to room temperature, smaller crystals with lower purity result. Suggestion: It is important to note that slow cooling is the key criteria for crystals to be formed in high purity. F. Further suggestions to the experimental procedures 1. It may take 15 – 25 minutes in step 18 to cool the mixture to room temperature 2. If time allowed, students can be asked to determine the melting point of the sample before recrystallization and compare with the data obtained from the crystal after recrystallization. G. Suggested answer to “Specific Chemical Hazards” Hazard Flammable Corrosive Toxic Irritant Harmful Oxidizing Explosive Others Chemical Acetic acid Aspirin Caffeine Dichloromethane Ethyl acetate Asphyxiant, Carcinogenic, CNS depression H. Reference 1. Saqib Ali, Abida Kalsoom, Journal of Chemical Education, 1991, 68, 877. 2. Irving Allan Kaye, Henry Yuska, Journal of Chemical Education, 1970, 47, 703. 3. Gene A. Hiegel, Journal of Chemical Education, 1986, 63, 273. 3