carboxylic acids and derivatives
advertisement

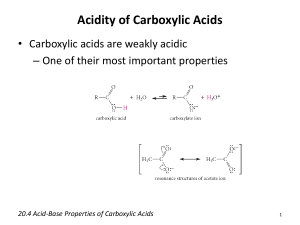
Unit 2 Module 1 Carboxylic acids and their derivatives CARBOXYLIC ACIDS AND DERIVATIVES page 1 of 8 Structure of a typical carboxylic acid This group is the functional group This is an example of an aliphatic acid Aromatic carboxylic acids have a carboxyl group attached directly to an aromatic ring. Reactions of carboxylic acids 1. With highly reactive metals and inorganic bases/alkalis to form the salt and hydrogen gas for highly reactive metals and a salt and water formed from inorganic bases 2CH3COOH(aq) + Na(s) ethanoic sodium acid CH3COONa(aq) + sodium ethanoate 2CH3COOH(aq) + NaOH(aq) ethanoic sodium acid hydroxide H2(g) hydrogen gas CH3COONa(aq) + H2O(l) sodium water ethanoate 2. Amide formation With ammonia to form an ammonium salt, which then decomposes by heat (remember this reaction is done in reflux) to form an amide RCOOH + NH3 RCOO-NH4+ RCOO-NH4+ RCONH2 + H2O Amides have the functional group below Unit 2 Module 1 Carboxylic acids and their derivatives page 2 of 8 3. Ester formation Reflux with alcohols in the presence of conc. sulphuric acid and 170 °C to form an ester and water (Note esters are low molecular mass compounds, which have sweet smells and are insoluble in water) RCOOH + R1OH conc.H2SO4 RCOOR1 + H2O 4. Acid chloride formation With chlorinating agents e.g. dichlorosulphur (IV) oxide SOCl2 or phosphorous (V) chloride PCl5 to form an acid chloride RCOOH PCl5 or SOCl2 RCOCl + HCl 5. With carbonates and hydrogen carbonates to give a salt, carbon dioxide and water 2RCOOH + Na2CO3 Acid chlorides have the following functional group O // --C \ Cl 2RCOO-Na+ + CO2 + H2O RCOOH + NaHCO3 RCOO-Na+ + CO2 + H2O 6. Reduction With LiAlH4 to form the corresponding alcohol RCOOH LiAlH4 (in ether) RCH2OH 7. Anhydride formation When 2 molecules of a carboxylic acid is reacted with a dehydrating agent such as phosphorous (V) oxide P4O10, a molecule of water is eliminated and an acid anhydride is formed. 2RCOOH distilled in P4O10 (RCO)2O Unit 2 Module 1 Carboxylic acids and their derivatives Checkpoint A page 3 of 8 Unit 2 Module 1 Carboxylic acids and their derivatives Reactions of acid derivatives page 4 of 8 1. Hydrolysis Acid chlorides are hydrolysed to give carboxylic acid and hydrogen chloride gas RCOCl + H2O RCOOH + HCl Esters are also hydrolysed to give the original alcohol and if the reagent is a strong mineral acid, then the carboxylic acid is formed RCOOR1 + H+ ROH + RCOOH If the reagent is a strong alkali, then the original alcohol is formed and the salt of the carboxylic acid RCOOR1 + OH- ROH + RCOO2. Ester formation Acid and aroyl chlorides react readily with alcohols and with phenols in alkaline solutions respectively to form esters CH3COCl (l) + C2H5OH (l) CH3COOC2H5 + HCl ethanoyl ethanol ethyl hydrogen chloride ethanoate gas CH3COCl + NaCl (s) sodium phenoxide O-Na+ O—C—CH3 + || O phenyl ethanoate 3. Amide formation Amides are formed by reaction of acid chlorides with ammonia and with primary and secondary amines RCOCl + NH3 RCONH2 + HCl RCOCl + R2NH RCONR2 + HCl 4. Anhydride formation When an acid chloride is heated with the sodium salt of a carboxylic acid, the acid anhydride is formed RCOCl + RCOO-Na+ (RCO)2O + NaCl NB acid anhydrides react in a similar way to acid chlorides; they form esters with alcohols and phenols and they form amides with ammonia and primary and secondary amines. Unit 2 Module 1 Carboxylic acids and their derivatives Checkpoint B page 5 of 8 ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… ………………………………………………………………………… Saponification (alkaline hydrolysis of fats/oils) When fats/oils are hydrolysed by an alkali, “soap” is produced. How is a fat/oil molecule formed? By the reaction of glycerol and long chain fatty acids (carboxylic acids with hydrocarbon chains containing 12 or more carbon atoms) Glycerol is propane-1,2,3-triol Long chain fatty acids could be: C15H31COOH palmitic acid (saturated), C17H35COOH stearic acid (saturated) C17H33COOH oleic acid (unsaturated) Unit 2 Module 1 Carboxylic acids and their derivatives page 6 of 8 When a fat/oil molecule is hydrolysis by an alkali, the glycerol is formed as well as the sodium salt of the long chain fatty acid. The sodium salt of the acid is the soap. Transesterification biodiesel production Biodiesel production is the act of producing the biofuel, biodiesel, through either transesterification or alcoholysis. The process involves reacting vegetable oils or animal fats catalytically with a short-chain aliphatic alcohols (typically methanol or ethanol). The vegetable oil is reacted with alkali in the presence of ethanol or methanol as a catalyst under reflux, which produces the esters of the fatty acids and glycerol. The biodiesel is the collection of the esters produced. Checkpoint C What advantages would there be for the use of biodiesel or biofuel in general? ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… ……………………………………………………………………………… …………………………………………………………………………....... Unit 2 Module 1 Carboxylic acids and their derivatives page 7 of 8 Explanation of relative acidities of acids and chlorine substituted acids Carboxylic acids are only partially ionised in aqueous solution owing to the predominantly covalent nature of the molecule are only weak in comparison with mineral acids. RCOOH + H2O RCOO- + H3O+ The relative strength of acids is attributed to the stability of the acid anion. The more stable the acid anion, the stronger the acid. The longer the hydrocarbon acid, the WEAKER the acid. However, the more electronegative atoms i.e. Cl atoms present in the aliphatic chain the STRONGER the acid. Acidic strength of aliphatic acids decreases in the order (Cl)3CCOOH > CH(Cl)2COOH > CH2ClCOOH > CH3COOH > CH3CH2COOH In aliphatic acids, the more electron withdrawing groups present, this represents a spreading of the charge on the oxygen atom (from the anion formed from dissociation in aqueous solution). Therefore trichloroethanoic acid is more acidic than dichloroethanoic acid etc. Propanoic acid is a weaker acid than ethanoic acid since there is an alkyl group (-CH3) which is electron donating and thus increasing the charge on the oxygen atom, making the anion formed from dissociation more unstable i.e. a weaker acid than ethanoic acid. NB The lower the pKa value, the stronger the acid. Note that aliphatic alcohols are the weakest acids, then phenols are stronger and finally carboxylic acids show the highest acidity. The acid anion of carboxylic acids are therefore the most stable, then the acid anion from phenols and finally the acid anion from ethanol. Unit 2 Module 1 Carboxylic acids and their derivatives Checkpoint C page 8 of 8