Beer's Law Lab: Determining Solution Concentration
advertisement

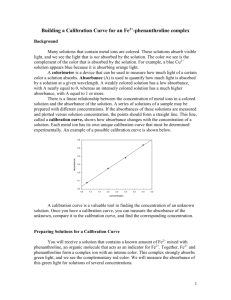
Determining the Concentration of a Solution: Beer’s Law Lab The primary objective of this experiment is to determine the concentration of an unknown “nickel (II) sulfate solution.” NOTE: Since NiSO4 is a suspected carcinogen, we will be using a safe green coloured substitute. However, throughout the lab, we will pretend that this green solution IS NiSO4. BACKGROUND: You will be using the Colorimeter shown in Figure 1. In this device, red light from the LED light source will pass through the solution and strike a photocell. The NiSO4 solution used in this experiment has a deep green color. A higher concentration of the green solution absorbs more red light and transmits less light than a solution of lower concentration. The computerinterfaced colorimeter monitors the light received by the photocell as either an absorbance (how much light didn’t go through) or a percent transmittance (how much light did go through) value. Figure 2 Calibration of the Colorimeter: The plastic cuvette, and even the water may absorb a little light. To correct for this, we calibrate the colorimeter. To calibrate, we use a “blank” (a cuvette with only water.). This “blank” is assigned an absorbance of 0.0. That way, any absorbance of red light only indicates how much green NiSO4 is present. Standard Solutions and the Graph You are to prepare 5 NiSO4 solutions of known concentration (standard solutions) by diluting a stock solution with various amounts of water. Each is transferred to a small, rectangular cuvette that is placed into the colorimeter. The amount of light that penetrates the solution and strikes the photocell is used to compute the absorbance of each solution. When a graph of absorbance vs. concentration is plotted for the standard known solutions, a directly proportional relationship (straight line graph) should result, as shown in Figure 2. For a coloured substance, Concentration Absorbance . (concentration is proportional to absorbance.)This relationship is known as “Beer’s Law”. (ie. The higher the concentration, the greater the absorbance) Determining Concentration of the Unknown Sample: The concentration of an unknown NiSO4 solution is then determined by measuring its absorbance with the colorimeter. Using the absorbance of the unknown solution, you can obtain its concentration by: a) reading its concentration off the graph b) calculating using the slope from the graph. eg. from graph, y = 1.23 x where slope = 1.23, y = Absorbance, x = concentration x = y/1.23 or concentration = Absorbance / slope Thus, the concentration of the unknown “NiSO4” is found using the student’s own graph. PRELAB PREPARATION: 1. Calculate the concentrations of the diluted solutions in trials #1-5 and fill in the concentration answers in your data chart. 2. Prepare a flowchart with diagram &/or words for the following procedure PROCEDURE Sample preparation (dilutions) 1. Label five clean, dry, test tubes 1-5 (One Partner does steps 2 & 3-water pipetting, the other partner does steps 4 & 5-NiSO4 pipetting:) 2. Put approximately 30 mL of distilled water in a 100 mL beaker. Rinse a pipette 3 times with distilled water. Discard the rinse water. Dry the beaker. 3.Add about 30 mL of distilled water to the 100-mL beaker. With cleaned pipet, deliver 8, 6, 4, and 2 mL of distilled water into Test Tubes 1-4, respectively. 4. Put approximately 30 mL of 0.40 M NiSO4 stock solution in a 100 mL beaker. Rinse a pipette 3 times with NiSO4 solution. Discard the rinse solution. Dry the beaker. 5. Add about 30 mL of 0.40 M NiSO4 stock solution to a 100-mL beaker Pipet 2, 4, 6, and 8 mL of 0.40 M NiSO4 solution into Test Tubes 1-4, respectively. Pour approximately 10 mL of NiSO4 into test tube 5. Rinse pipet 3 times with water before returning it to the rack. 6. Thoroughly mix each solution with a stirring rod. Clean and dry the stirring rod between stirrings (Why?). TWO GROUPS OF STUDENTS WILL TAKE TURNS USING THE COLORIMETER. FIRST GROUP TO FINISH THEIR DILUTIONS WILL CALIBRATE AND USE THE COLORIMETER FIRST. 2ND GROUP WILL GET THE CALIBRATED CUVETTE FROM THE FIRST GROUP AND SKIP THE CALIBRATION Checkpoint: What is the total Vsolution in each test tube? Why rinsing pipette 3 times in step 2? Computer set-up for first group to use the colorimeter: Obtain a Colorimeter and plug it into a channel of the Lab Quest using the adapter (for the Box style). Plug the Lab Quest into the wall socket and turn it on. Use the stylus to select: Sensors→Sensor setup On the appropriate Channel, drop down the sensor menu and select: Colorimeter You should now have a live Absorbance display on the screen. (Not Transmittance) You are now ready to calibrate the colorimeter. (NOTE IF YOU ARE THE SECOND GROUP TO USE THE COLORIMETER, YOU CAN SKIP THIS SECTION AS LONG AS YOU USE THE CUVETTE & CALIBRATION USED BY THE PREVIOUS GROUP ) 7. Prepare a blank by filling a cuvette 3/4 full with distilled water. To correctly use a colorimeter cuvette, remember: • All cuvettes should be wiped clean and dry on the outside with a tissue. • Handle cuvettes only by the top edge of the ribbed sides. • All solutions should be free of bubbles. • Always position the cuvette with its reference mark facing toward the white reference mark at the right of the cuvette slot on the colorimeter. Use the SAME cuvette for the calibration and ALL the solutions Place the blank cuvette in the cuvette slot of the colorimeter and close the lid. CALIBRATION FOR NEWER OVAL STYLE COLORIMETERS: 8. Select the red light (635 nm) using the arrow keys on the colorimeter. CALIBRATION FOR OLD BOX STYLE COLORIMETERS: 8. Experiment Sensor SetupCalibratePerform Now. Turn the wavelength knob of the colorimeter to the 0%T position. In this position, the light source is turned off, so no light is received by the photocell. Type “0” in the % edit box. When the displayed voltage reading for Input 1 stabilizes, click Keep . 9. Press and hold down the CAL button on the colorimeter to begin the calibration. Release the button when the red LED begins to flash. The calibration should now be complete and absorbance should read 0.000 or 0.001 9. For Reading 2, turn the wavelength knob of the colorimeter to the Red LED position (635 nm). In this position, the colorimeter is calibrated to show 100% of the red light being transmitted through the blank cuvette. Type “100” in the % edit box. When the displayed voltage reading for Input 1 stabilizes, click Keep , then click OK . Keep the Red setting for the rest of the experiment. Collecting Absorbance Data on your samples 10. You are now ready to collect absorbance data for the five standard solutions. Empty the water from the cuvette. Using the solution in Test Tube 1, rinse the cuvette twice with very small amounts of solution and then fill it 3/4 full. Wipe the outside with a tissue and place it in the colorimeter. After closing the lid, wait a few seconds for the absorbance value displayed on the monitor to stabilize. Then record the Absorbance on the lab sheet. 11. Discard the cuvette contents . Rinse the cuvette twice with the Test Tube 2 solution, and fill the cuvette 3/4 full. Wipe the outside, place it in the colorimeter, and close the lid. When the absorbance value stabilizes, record the Absorbance on the lab sheet. 12. Repeat the Step 12 procedure using test tubes 3 , 4 and 5 and the vial with your unknown sample, Recording the Absorbance of each on your lab sheet. Record the unknown sample number on the vial, rinse and return it to the teacher’s container at the front. 13. If there is another group waiting for the colorimeter, allow the next group to use the colorimeter and the cuvet. DO NOT CLOSE THE PROGRAM OR THE CALIBRATION WILL BE LOST. Graphing of your Known Test tubes #1-5 Results in Excel 14. On the computer, prepare your graph on Excel as per the separate instructions. (see other side of this page for example). Make sure: NiSO4 is included in a descriptive title, graph is a proportional fit (straight line that goes through zero), equation for the line is shown on the graph.) 15. Estimate from reading off your graph, the concentration of your unknown sample and record below. On your lab sheet record the equation for the line. 16. Print a HARD COPY (no email accepted) of your graph per person in the group. 17. Finish the lab calculations to determine the concentration of your unknown and hand in your lab sheet at the end of class. data/graph /5 unknown sample accuracy Lab sheet calculations /3 prelab Quiz 8 multiple choice questions /3 total /8 /19