AP Chemistry: Energy Levels & Electronic Transitions Lab
advertisement

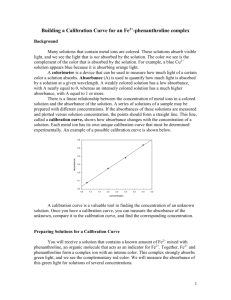
Name _____________________________________________ Lab W 9/3-Th 9/4, due by Th 9/11, not accepted late AP Chemistry Investigation 1: Energy Levels and Electronic Transitions Experimental Objective: To use colorimetric methods to produce Beers’ Law plots for colored transition metal cation solutions, and then to use those plots to quantitatively analyze a solution containing one of the cations in an unknown concentration. Concepts: electromagnetic spectrum, ground and excited states, spectrophotometry, quantitative analysis Introduction Many chemical substances have distinctive colors. o Copper (II) salt solutions are blue or blue-green o Potassium permanganate is dark purple o Iron (III) salt solutions are yellow Niels Bohr suggested that energy levels for the electrons on atoms were quantized, due to the observation of the bright-line spectra that each element exhibits. His interpretation of these spectra was that atoms were absorbing energy and being stimulated from their ground state to some excited state. When they return to ground state, they release photons—and the energy of these photons exactly matches the energy difference between the two states. Excluding elements in the zinc family, all transition metal ions have electrons in incomplete d-orbitals. Ordinary visible light (which consists of a continuous range of energies) is able to stimulate some of these delectrons to excited states; the electrons in these d-orbitals absorb certain energies of light to make these transitions. Each ion absorbs only specific energy amounts corresponding to specific electron “jumps” from the ground state to the excited state; any other color/energy of light simply passes through. For example, copper (II) solutions appear blue because they do not absorb blue light. Instead, it absorbs light of the complementary color spectrum, such as orange/red. We distinguish between electromagnetic radiation of different energies using frequency (ν) and wavelength (λ). In this experiment, we will use wavelength. The higher the energy of the wave, the higher the frequency, and the shorter the wavelength. We will test solutions of three common transition metal cations: cobalt (II), copper (II), and nickel (II)— Co2+, Cu2+, and Ni2+ respectively. One group will choose one of these metals cations. The maximum group size is four students, so some groups may use the same metal cation. You will test each solution with three different colors of light to see which color is most strongly absorbed by each. o Red λ = 635 nm o Green λ = 590 nm o Blue λ = 470 nm You will also vary the concentrations of the ion solutions to see how the amount of light absorbed varies with concentration; your diluted concentrations are up to you. This relationship is described by the Beer-Lambert Law, or simply Beer’s Law. You will make a Beer’s Law plot (absorption vs.concentration) and use your plot to determine the concentration of an unknown solution of your chosen ion. I will know the actual concentration; you will compare your experimental value to the true value and calculate your percent error. Too long, didn’t read: your group will choose a solution of one ion and make several dilutions of it. The amount of three colors of light that each dilution absorbs will be measured. A plot will be made from this data. Lastly, you will measure the absorption of a solution of your ion of unknown concentration, and use your plot to experimentally determine its concentration. The true answer will be revealed, and you will find your percent error. First Day Th 9/4: after we go over the test, you will have the rest of class to set up your experiment. Get as far as you can—you could make your dilutions and familiarize yourself with Logger Pro (on my computer) as well as the equipment we will use. You will make four dilutions of your sample starting with a 0.5 M standard which I will prepare. Make your data table in your “lab notebook” before we do the lab on F 9/5. Here’s a sample table. Note that it is up to your group to pick a dilution factor and figure out how to make your dilutions. Concentration (M) 0.5 Red Absorption Green Absorption Blue Absorption Pre-lab Questions (completed before our lab, answered in your lab notebook) 1) Write the full or noble gas electron configurations for the ions used in this experiment. a. Cobalt (II) b. Nickel (II) c. Iron (II) 2) Determine the concentrations you would like to use for your four dilutions and draw a diagram or write a few sentences explaining how you will make them. P.S.—our lab manual recommends making 0.4 M, 0.3 M, 0.2 M, and 0.1 M as your concentrations, but you can use different concentrations if you would like. Second Day F 9/5: doing the actual lab (record all data in your lab notebook in neat, organized tables) 1) Obtain 20 mL of your 0.50 M solution and create your four dilutions. Be very careful (accurate/precise), since your data will suffer if your measurements are not accurate. Make sure to leave some of the 0.50 M standard, since you will test it as well. 2) Measure the width of one cuvette. You will need this for your Analysis. 3) You will add a few mL of each solution to a cuvette. The cuvettes are placed into the colorimeter, and then the absorption is “read”. This part is simple but don’t mess up. a. The colorimeter must be calibrated before it is used. We will use water as a “blank”. I intend to do this part…you should probably watch so you can see how easy it is to use the colorimeter. b. Don’t get your finger prints on the smooth sides of the cuvette. If you do, you will need to clean them. Use a separate cuvette for each dilution. c. Don’t spill anything into the colorimeter. d. Make sure you have the right solution in the colorimeter and that your colorimeter is set to the correct color before you record each reading. e. I have one computer so we will use one colorimeter. Don’t take too long, others will be waiting. 4) After you have collected the data for each of your dilutions for each of the three colors, you will be given a solution of your ion of unknown concentration. Record its absorption for all three colors. When you are finished, ask me for the true concentration of your unknown, which you will need for the Analysis section. 5) The waste for this lab can be disposed of in the sink. Wash all of your glassware, as well as the cuvettes, and return them to the proper places. 6) Note: this lab can continue into M 9/8 if necessary. It is more important to get good data than to rush. Analysis and Conclusions (answered in your “lab notebook”, write in full sentences and give thorough explanations) 1) Use graph paper or a spreadsheet (ex. Excel) to plot the data for all three wavelengths. Plot all three on the same graph, but use different colors for each line (red for red, etc.). Make sure to put the correct variables on the correct axes…check with your group or ask me if you are not sure. Absorbance has no units, and concentration is in M (molarity, or molar). 2) In complete sentences, explain/estimate (showing your calculations for b and c) a. The color of light that your metal cation absorbs the most strongly (specify the wavelength, λmax). b. The absorbance of a 0.33 M solution c. The concentration of a solution with an absorbance of 0.150 as its λmax 3) Compare your data with the other groups and a. Determine which metal cation has electons that undergo the greatest energy change when they absorb light b. Look up the true value for each cation and explain if the class’s general results were correct 4) Beer’s Law is written as A = ξlc, where A = absorbance (unitless, or “absorbance units” AU), l = path length (cm), and c = solution concentration in molarity. The second term, ξ, is called the extinction coefficient (AU/cmM). a. Choose three of your dilutions for one color (red, blue, or green). Calculate the extinction coefficient for each and average this value. Show all of your calculations for credit. b. You should have found that the three lines in your plot are linear. Rearrange Beer’s law to explain why. 5) Estimate the concentration of your unknown. Explain how you came to this conclusion (hint: graph). 6) Compare your value from 5) with the true value, and calculate your percent error.