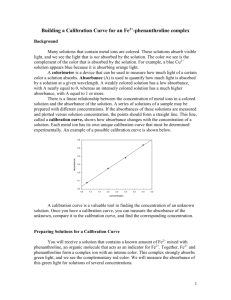
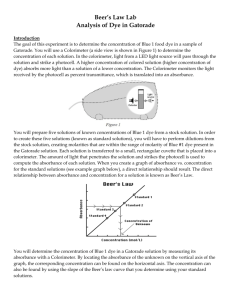
Concentration
advertisement

Beer’s Law: Using a Colorimeter Introduction: In this experiment, the concentration of an unknown cobalt (II) nitrate solution will be determined using the colorimeter. A green light from the LED light source will pass through the solution and strike a photocell. The Co(NO3)2 solution used in this experiment has a pink color; higher concentrations absorb more light than solutions of lower concentrations. Four Co(NO3)2 solutions will be tested; the resulting data will be used to determine the concentration of 2 unknown solutions. When a graph of absorbance vs. concentration is plotted, a straight line should be generated, indicating a direct relationship. This relationship is known as Beer’s Law. The concentration of an unknown solution is determined by measuring its absorbance and finding the corresponding concentration from the graph. Purpose: The purpose of this experiment is to use the colorimeter and Beer’s Law to determine the concentrations of unknown solutions. Equipment/Materials: laptop serial box interface LoggerPro software Vernier Colorimeter printer plastic rectangular cuvettes w/ lids Kimwipes standard Co(NO3)2 solutions unknown Co(NO3)2 solutions droppers Safety: Always wear goggles in the lab. Do not spill any solutions on your skin; inform the instructor of any accidents. Procedure: 1. Double click on the LoggerPro icon. Go to the File menu and select Open. Open Chemistry with Computers, then open Experiment 11. Choose Exp 11 Colorimeter. 2. Plug the colorimeter into Port 1 on the Serial Box. 3. Go to the Experiment menu and choose Calibrate. Click Perform Now. With the sample compartment empty, close the lid. Turn the wavelength knob of the colorimeter to the 0% T position. (The light source will be turned off, so no light is received by the photocell.) The number 0 should appear in the % edit box. When the voltage reading stabilizes, click Keep. 4. Prepare a blank by rinsing a cuvette with distilled water. Fill the cuvette ¾ full of distilled water. Remember: Cuvettes should always be wiped on the outside with a Kimwipe. Hold the cuvettes by the top edge of the ribbed sides. Solutions should be free of bubbles. Position the cuvette with the clear side in the light path. 5. For reading 2, turn the wavelength knob of the colorimeter to the Green LED position (565 nm) and place the blank in the colorimeter. The colorimeter will be calibrated to show 100% of the green light being transmitted through the blank cuvette. The number 100 should appear in the % edit box. When the voltage has stabilized, click Keep, then click OK. 6. Remove the blank cuvette. Rinse the cuvette twice with ~1 mL portions of the lease concentrated standard solution to be tested. Fill it ¾ full with the solution, and wipe with a Kimwipe. Place the cuvette in the colorimeter and close the lid. 7. Click Collect. Wait for the absorbance value to stabilize, then click Keep. Type the concentration in the edit box and press Enter. The data point should now be plotted on the graph. 8. Remove the cuvette and rinse twice with ~1 mL portions of the next standard solution. Wipe the cuvette, place it in the colorimeter, and close the lid. When the absorbance stabilizes, click Keep, type the concentration in the edit box, and press Enter. 9. Repeat step 7 for all standard solutions. When finished, click Stop. 10. Record the absorbance and concentration data pairs in your data table. 11. Rinse and fill a cuvette with an unknown solution. Wipe the cuvette, place it in the colorimeter, and close the lid. Record the absorbance value in your data table. Do not click collect! 12. Repeat step 10 for all unknowns. 13. Click on the graph of absorbance versus concentration. To see if the curve represents a direct relationship between these two variables, click the Linear Regression button, which is the third button from the right. A best fit or linear regression line will be shown for your data points. 14. If instructed to print the graph or data table, click on the appropriate window. Go to the File Menu and select Print. Enter your names and print the graph. Data: Concentration (M) Unknown Number Absorbance Absorbance Concentration Calculations: 1. Use the following method to determine the unknown concentration. With the linear regression curve still displayed on your graph, choose Interpolate from the Analyze menu. A vertical cursor will appear on the graph. The cursor’s x and y coordinates are displayed at the bottom of the floating box. Move the cursor along the regression line until the absorbance (y) value is approximately the same as the absorbance value recorded for the unknown. The corresponding x value is the concentration of the unknown in Molarity. Record this value in the data table. 2. If instructed, print this screen following the procedure from step 13. 3. Optional second method of determining the concentration: Remove the regression line by clicking on the corner of the floating box so that the box and regression line disappear. Go to the Analyze menu and choose Curve Fit. Select Proportional (y=Ax). Click on Try Fit and then OK. Go to the Analyze menu and select Interpolate. Determine the concentration as you did in step 1. Record the concentration in the Data Table. Place this value in parentheses beneath that recorded in step 1. Compare the values. 4. If instructed, print this screen as in step 13 of the procedure. 5. Repeat the calculations for all unknowns. Questions: 1. What does absorbance mean? How does it compare to transmittance? 2. Should the line in the standard curve pass through the origin? Why or why not? Source: Chemistry with Computers, Holmquist, D. and Volz, D., Vernier Software, 1997, pages 11-1 11-2T (with slight adaptations for Advanced Instrumentation Workshop 2000).