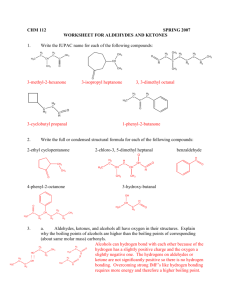
CHM 266 Experiment 2 Handout
advertisement

CHM 266 Experiment 4 Handout Free Radical Chlorination Spring 2006 Reference: J. A. Moore, D. L. Dalrymple, O. R. Rodig, "Experimental Methods in Organic Chemistry, 146, 3rd Ed., Saunders College Publishing, Philadelphia, 1982. The experiment was modified from this reference. Portions of the handout are direct quotes from it. Introduction This experiment involves a reaction that is covered in Chapter 4 Radical Reactions of the CHM 261 course. Actually, this is exactly the type of experiment that led chemists to come up with the mechanism and principles of radical reactions that will be described in lecture Reaction and Mechanism Although saturated hydrocarbons are inert to most acidic and basic reagents, they can be "halogenated" in the presence of what is called a free radical initiator. As shown for cyclohexane, a chlorine replaces a single hydrogen. As described in lecture, the number of hydrogens replaced will depend on the conditions. This type of halogenation occurs by a mechanism known as a radical chain reaction. The mechanism is provided below the reaction. Cl Cl2, h + HCl excess Initiation h Cl 2 Cl Cl Propagation Cl + H R + Cl R Cl Cl R H + R Cl + Cl Termination R + Cl R + R R R Cl R Chlorine gas is toxic, corrosive, and can be difficult to handle on the bench top scale. A safer method uses sulfuryl chloride and benzoyl peroxide (yes, the zit medicine). At relatively low temperatures, the O-O bond breaks to form benzoate radicals. O O O O O Ph Ph 2 O Ph benzoyl peroxide Ultimately this leads to the formation of other radicals generically represented as R’. The chlorine atoms needed for the reaction are then generated in the following reaction. O R' + Cl S Cl R Cl + SO2 + Cl O Safer doesn't mean without hazard. Sulfuryl chloride is also toxic and corrosive. The carbon tetrachloride that is used as a solvent for the sulfuryl chloride is carcinogenic. Benzoyl peroxide is an explosion hazard. The SO2 generated in the reaction is toxic. After all this, it may be hard to believe that this is safer, but it is. You will be working with such small amounts that someone would have to be fairly careless to hurt themselves or anyone else. Of course being careless means working with most of this material outside the hoods. When a molecule contains more than one type of hydrogen atom (i.e. 1, 2, or 3), as in 2-methylbutane, a mixture of alkyl chlorides is obtained. Even if only monochlorination occurs, there should be four alkyl chloride products. If you look at the 2-methylbutane structure shown, the H atoms are labeled as 1, 2, 3, or 4. The products obtained from replacing those H atoms are labeled as A, B, C, and D respectively. Assuming that the H atoms are equally reactive, then the H1's should be 6 times more likely to be replaced as H2 since there are 6 H1's and only 1 H2. This would be the statistical prediction. The table below the reaction shows how much of each isomer should be formed based on the statistical prediction. Then it shows how much of each actually formed. It doesn't match. H3C H3C CH Cl H3C 1 H3C 1 C H2 C H2 2 C H2 3 Isomer Statistical prediction % Observed % CH 3 H3C Cl H3C 4 CH H3C C C H2 Cl A Cl2, h CH CH 3 CH 3 B H3C C H CH CH 3 C H2 H3C C C H2 Cl D A 50 B 8 C 17 D 25 34 22 28 16 Too little of the primary chlorides are obtained, and too much of the secondary and tertiary. That's because the H atoms don't react at equal rates. The difference in reactivity has to do with radical stability (in Chapter 4). Based on these results and many other experiments, it was determined that the relative rates of "radical" reaction with hydrogens 1 through 4 (abstraction) are 1.0:4.0:2.5:1.0. The order of reactivity is tertiary > secondary > primary. To test whether these ratios are applicable to other hydrocarbons, you will perform the chlorination reaction on 2,4-dimethylpentane using sulfuryl chloride and benzoyl peroxide to generate chlorine atoms. The product mixture will be analyzed by GC. Procedure Note the hazards listed in the previous section. Also, measure the benzoyl peroxide by carefully pouring onto weighing paper. Do not use a metal spatula or scoopula. Read the notes at the end of the procedure. Heat a 400 ml beaker on a steam bath to 75 C. (It may be necessary to heat the water on a hot plate with stirring to ~ 65 C and then transfer to the steam bath to save time.) Be sure to insert the thermometer to its immersion line. Obtain 1 ml of the sulfuryl chloride in carbon tetrachloride solution with a 1 ml pipet (.32g in 1 ml solution) and place it in a clean dry 25 ml round-bottom flask. To this solution, add by pipet 0.5 ml of 2,4-dimethylpentane with a 0.5 ml pipet (d = 0.67). Add 25 mg (0.025 g) of benzoyl peroxide to the mixture. Add a boiling stone and attach a reflux condenser. Place a drying tube containing KOH pellets on top of the condenser to exclude moisture and absorb HCl. Clamp the apparatus in the water bath so that about 1/2 of the flask is submerged. Heat the bath at such a rate that the temperature remains 75 2 C for 30 minutes. Transfer the contents to a test tube. Add 3 ml of water, stir, and then add 1 ml of saturated sodium bicarbonate solution. Shake or stir the contents well. Then transfer the carbon tetrachloride layer (CCl4 is more dense than water) to another test tube. Be sure not to transfer any water. Add a few grains of anhydrous sodium sulfate to dry the sample. Remove a portion of this sample without transferring any of the grains of sodium sulfate. Analyze this mixture by GC. Notes 1. To save time some shortcuts will be made. If this were a research project on experiments that had never been published, we would probably not take the shortcuts. a. We will not use known samples to determine retention times. The order of elution should of the compounds is (air), 2,4-dimethylpentane, carbon tetrachloride, 2-chloro-2,4-dimethylpentane, 3-chloro-2,4-dimethypentane, and 1-chloro-2,4-dimethylpentane. b. Response factors will not be calculated. c. The last two peaks in the chromatogram may not be resolved although we will use the data. 2. The proper attenuation will be announced. 3. Let the sample run for 10 minutes. 4. Be sure to place the GC conditions, retention times, peak areas, and area % values in your notebook data section. After the report is returned, staple the chromatogram in your notebook also. 5. Tiny granules of sodium sulfate that cannot be seen by the naked eye may nonetheless plug the syringe needle. To avoid this, rinse the syringe with acetone, then water twice, then acetone, and then 2,4-dimethylpentane, and 5 times with your sample before injection. Finally rinse the syringe with acetone, twice with water, and finally acetone before turning in the syringe.