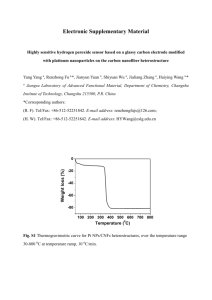
Hydrogen Peroxide Decomposition Reaction Kinetics
advertisement

Hydrogen Peroxide Decomposition Reaction Kinetics Purpose: In this experiment, you will determine the rate law and activation energy for the iodidecatalyzed decomposition of hydrogen peroxide. Overview: The decomposition of hydrogen peroxide is catalyzed by iodide according to the following reaction: I 2H2O (l) + O2 (g) 2H2O2 (aq) The speed of the reaction is determined from the reactants being consumed or from products that are being formed. This must be determined experimentally by measuring the rate of change in the concentration of one of the reactants or one of the products. The change of concentration can be measured by such physical properties such as the volume of a gas or color intensity of a solution. The rate may be expressed, for example, as moles per liter of product being formed per minute, milliliters of gas being produced per minute, or moles per liter of reactant being consumed per second. During this experiment, you will determine the rate of decomposition of hydrogen peroxide in the presence of a catalyst, iodide. The goal in this experiment is to deduce a rate law for the reaction, showing the dependence of the rate on the concentrations of H2O2 and I-. Your rate law will be of the form: - [ H 2 O 2 ] = k[H2O2]x[I-]y t k is the reaction rate constant and depends only on temperature. x is the reaction order with respect to the hydrogen peroxide concentration and y is the reaction order with respect to the iodide ion concentration. Your objective is to determine the numerical values for the exponents x and y and rate constant, k. You will also study the effect of temperature on the reaction. Materials: 0.25M KI solution 3% H2O2 solution distilled water 125mL or 250mL Erlenmeyer flask 50mL gas collection tube ring stand test tube clamp one-hole rubber stopper beaker water bath 50mL graduate cylinder 5 and 10 mL pipets thermometer Procedure: 1. Fill one beaker about half full with water. Fill the gas collection tube with water and invert into this beaker. Clamp the gas collection tube to the ring stand. You will use this to measure the volume of gas generated in the reaction. 2. Place the Erlenmeyer flask into a water bath. Fill the waterbath two thirds full of water. Record the temperature of the water. Cap the Erlenmeyer flask with a onehole rubber stopper. Insert a short piece of glass tubing into the one-hole rubber stopper. If necessary, cut a piece of glass tubing and fire polish the ends. Connect one end of the rubber tubing to the glass tubing and insert the other end into the gas collection tube. You are now ready to start the reaction. 3. Remove the rubber stopper from the 50 mL flask. Add 10 mL of the 0.25 M KI solution and 15 mL of distilled water to the flask. 4. Add 5 mL of 3% H2O2 to the Erlenmeyer flask. Swirl to mix the solutions and immediately replace the rubber stopper. Begin taking oxygen volume readings immediately. 5. Record the time and oxygen volume (mL) every ten seconds for 240 seconds or 30mL (whichever comes first). Swirl the flask during the reaction to prevent the solution becoming super saturated with oxygen. 6. Repeat the experiment, using a clean flask, with10mL of the 0.25-M KI solution and 10mL of distilled water, then adding 10 mL of the 3% H2O2. 7. Repeat the experiment again, this time using 20mL of the 0.25-M KI solution and 5mL of distilled water, then adding 5 mL of the 3% H2O2. 8. Replace the water in the water bath containing the Erlenmeyer flask, with water that is 10-20C warmer than previously used. Repeat the experiment using 10mL of 0.25M KI, 15mL of distilled water and 5mL of the 3% H2O2. 9. For each of the three trials, plot the volume of oxygen in milliliters versus the time in seconds. Fit the data with the best-fit curve or straight line for each trial, ignoring the first sixty seconds of data. (Do not draw a line that connects point to point.) 10. Calculate the slope (mL/sec) of each line. The slope of each line gives the rate of oxygen production in mL/seconds. 11. Use the slopes and the details from each trial to determine the reaction orders for the I- and H2O2. Note that the KI and H2O2 volume are proportional to their concentrations in the reaction solution. Slope (mL/sec) KI 10mL 10mL 20mL Trial 1 Trial 2 Trial 3 H2O2 5mL 10mL 5mL 12. Calculate the rate constant, k, for the equation: - [ H 2O2 ] = k[H2O2]x[I-]y t Substitute values for [H2O2], [I-], x,y and - [ H 2 O 2 ] / t into the equation and solve for k. Use the reaction orders determined above for x and y. Using data from one of the trials, calculate the molarity of the H2O2 and I- in the reaction solution and the hydrogen peroxide disappearance rate. Use these values to substitute into the above equation. You can determine the hydrogen peroxide disappearance rate from the rate of oxygen production. Convert the rate of oxygen production to moles per second using PV=nRT. Remember to reduce the pressure of the oxygen by the water vapor pressure. Use stoichiometry to convert moles of oxygen to moles of hydrogen peroxide. Use the solution volume to convert the moles per second to molarity per second. Calculate the rate constant, k, to two significant digits. Be sure to include units. 13. Examine your results to determine the effect that temperature had on the reaction rate. 14. Calculate k for the higher temperature, and, using both k values determine the activation energy for this reaction1. 1 Activation energy, Ea, is related to the reaction rate constant, k, by the Arrhenius equation: k=Ae -Ea/RT. R is the ideal gas constant, 8.314510 J/(K•mol). A is the frequency factor with units of L/mol •s, and is related to the fraction of collisions that have the correct geometry. The activation energy can be determined using reaction rate constants from two different temperatures using the Arrhenius Equation rearranged as: ln k2 – ln k1 = – Ea R 1 1 T2 T1 Questions: 1. How would your calculated reaction rate constants and calculated activation energy have been affected if the nominally 3% hydrogen peroxide had a concentration of only 2%? 2. How would your results have been affected if extra water had accidentally been added to the reaction mixture? 3. If you do not agitate the reaction solution, it can become supersaturated with oxygen. How would this affect your results? 4. If you had been able to directly determine the concentration of hydrogen peroxide in the reaction solution, you would have been able to graph the concentration versus time. What would that graph look like? 5. If you had been able to directly determine the concentration of iodide in the reaction solution you would have been able to graph its concentration versus time. What would that graph look like? 6. What would you graph versus time to determine the reaction rate constant? How would you calculate k from the graph? adapted from a lab at Occidental College http://departments.oxy.edu/tops/Kinetics/kinetics.pdf