Electronic Supplementary Material
advertisement
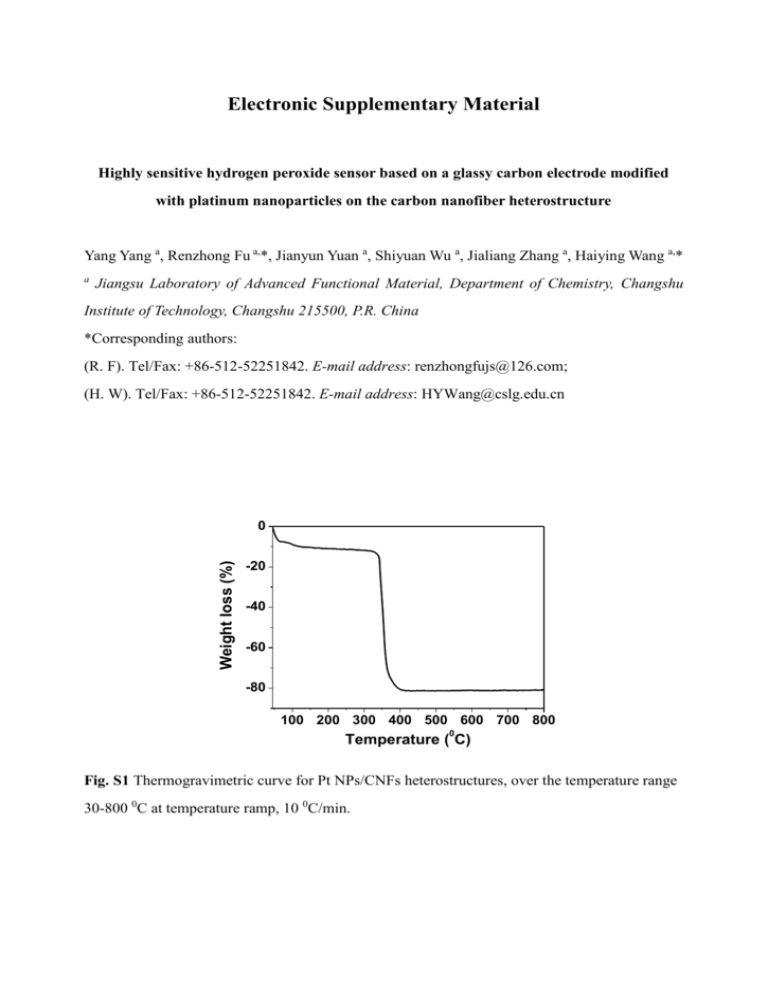
Electronic Supplementary Material Highly sensitive hydrogen peroxide sensor based on a glassy carbon electrode modified with platinum nanoparticles on the carbon nanofiber heterostructure Yang Yang a, Renzhong Fu a,*, Jianyun Yuan a, Shiyuan Wu a, Jialiang Zhang a, Haiying Wang a,* a Jiangsu Laboratory of Advanced Functional Material, Department of Chemistry, Changshu Institute of Technology, Changshu 215500, P.R. China *Corresponding authors: (R. F). Tel/Fax: +86-512-52251842. E-mail address: renzhongfujs@126.com; (H. W). Tel/Fax: +86-512-52251842. E-mail address: HYWang@cslg.edu.cn Weight loss (%) 0 -20 -40 -60 -80 100 200 300 400 500 600 700 800 0 Temperature ( C) Fig. S1 Thermogravimetric curve for Pt NPs/CNFs heterostructures, over the temperature range 30-800 0C at temperature ramp, 10 0C/min. Fig. S2 Current-time responses of the Pt/CNFs-GCE on successive injection of 0.1 mM H2O2 (a) and 0.5 mM H2O2 (b) into N2-saturated PBS (0.1 M, pH 7.0). Cyclicity of the Pt/CNFs-GCE after scanning 50 cycles continuously in 0.1M phosphate buffer solution (pH 7.0) added 2.0 mM H2O2 with scan rate of 100 mV·s−1 from -1.0 V to1.0 V (c). Amperometric responses of 0.2 mM five relevant electroactive species and 0.1 mM H2O2 on the Pt/CNFs-GCE at -0.2 V (d). Table S1 Comparison of nonenzymatic H2O2 sensors CuO2-rGOa Linear Detection range limit (mM) (µM) 0.01-13.1 1.6 8 0.03-12.8 21.7 PBI-BA-Gsb Cu2O NGs/GNsc Cu NCs Graphene-AuNP/GCE 0.025-5 0.3-7.8 0.01-1.0 0.02-0.28 3.1 20.8 10 6 Response R2 Stability time (s) 1.5 0.9994 81.6 % (14 days) 2 -92 % (14 days) 1.4 0.996 -7 0.9978 -----0.994 -- NP-PdCr 0.1-1.9 3.1 1.5 Electrode materials CuO-SiNWs [7] [1] [2] [3] [4] [5] [6] 0.0075-1 1.0 0 Pt/MWCNTs-PANI-G 0.007-2.5 2.0 CEe CoNP/Pt/CNTs/GCE 0.0002-1. 0.1 25 PVA-MWCNTs-PtNP 0.002-3.8 0.7 s/GCEf 5 93.1% (14 days) 0.9980 -- 5 0.9996 -- [9] 2 0.9979 -- [10] -- -- [11] Pt/CNFs-GCE 2 Pd/MCNsd 0.005-15 1.7 0.996 References 87 % (1 month) 0.9994 98.1 % (14 days) [8] This work Abbreviation: aCuO2-rGO, CuO2-reduced graphene oxide. bPBI-BA-Gs, poly[N-(1-onebutyric acid)benzimidazole] / graphene sheets. cCu2O NGs/GNs, Cu2O nanocubes/graphene nanosheets. d MCNs, mesoporous carbon nanospheres. eMWCNTs, Multi-wall carbon nanotube. fPVA, poly(vinyl alcohol). References 1. Huang JF, Zhu YH, Zhong H, Yang XL, Li CZ (2014) Dispersed CuO Nanoparticles on a silicon nanowire for improved performance of nonenzymatic H2O2 detection. ACS Appl Mater Inter 6:7055-7062 2. Xu FG, Deng M, Li GY, Chen SH, Wang L (2013) Electrochemical behavior of cuprous oxide–reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim Acta 88:59– 65 3. Hua MY, Chen HC, Tsai RY, Leu YL, Liu YC, Lai JT (2011) Synthesis and characterization of carboxylated polybenzimidazole and its use as a highly sensitive and selective enzyme-free H2O2 sensor. J Mater Chem 21:7254-7262 4. Liu MM, Liu R, Chen W (2013) Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron 45:206-212 5. Hu LZ, Yuan YL, Zhang L, Zhao JM, Majeed S, Xu GB (2013) Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta 762:83– 86 6. Hu JG, Li FH, Wang KK, Han DX, Zhang QX, Yuan JH, Niu L (2012) One-step synthesis of graphene–AuNPs by HMTA and the electrocatalytical application for O2 and H2O2. Talanta 93:345– 349 7. Zhao DY, Wang ZH, Wang JP, Xu CX (2014) The nanoporous PdCr alloy as a nonenzymatic electrochemical sensor for hydrogen peroxide and glucose. J Mater Chem B 2:5195-5201 8. Bian XJ, Guo K, Liao L, Xiao JJ, Kong JL, Ji C, Liu BH (2012) Nanocomposites of palladium nanoparticle-loaded mesoporous carbon nanospheres for the electrochemical determination of hydrogen peroxide. Talanta 99:256–261 9. Zhong HA, Yuan R, Chai YQ, Zhang Y, Wang CY, Jia F (2012) Non-enzymatic hydrogen peroxide amperometric sensor based on a glassy carbon electrode modified with an MWCNT/polyaniline composite film and platinum nanoparticles. Microchim Acta 176:389–395 10. Han LJ, Wang Q, Tricard S, Liu JX, Fang J, Zhao JH, Shen WG (2013) Amperometric detection of hydrogen peroxide utilizing synergistic action of cobalt hexacyanoferrate and carbon nanotubes chemically modified with platinum nanoparticles. RSC Adv 3:281–287 11. Fang YX, Zhang D, Qin X, Miao ZY, Takahashi S, Anzai J, Chen Q (2012) A non-enzymatic hydrogen peroxide sensor based on poly(vinyl alcohol)–multiwalled carbon nanotubes-platinum nanoparticles hybrids modified glassy carbon electrode. Electrochim Acta 70:266–271











