Light
advertisement

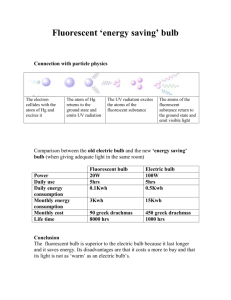
Making Light Light is a kind of radio wave. It differs in being much higher frequency than the waves used in radio, TV, microwave ovens, or cell phones. For example, the range of FM stations is from 92 megahertz to 107 megahertz ("hertz" is an abbreviation for "number of times per second", so this means that the FM radio waves are vibrating at a frequency of about 100,000,000 times per second); visible light is 10^15 hertz, which is ten million times faster. Visible light is a small part of the radio spectrum. • Infrared is lower frequency than visible light • Red light is low frequency • Blue light is higher frequency • Ultraviolet is higher frequency than visible light The frequency of blue light is about twice the frequency of red light. One way to make light is to get an object hot. The power emitted and the frequency range depend on the temperature. All objects emit light, but near room temperature the amount radiated is small and it is all infrared light that our eyes cannot see. Only above 500 C does an object begin to emit visible light. A rough translation of color into temperature is as follows: • Red hot == 500 C • Yellow hot == 1000 C • White hot (like the sun) == 6000 C The sun is a very hot object – hotter than we can make anything on earth without having it vaporize. An incandescent light bulb is about 1500 C, just below the melting temperature of tungsten. So the "white light" made by ordinary light bulbs is actually a bit orange. The picture shows how much light of each frequency arrives at the surface of the earth from the sun. The "teeth" cut into it are where water and carbon dioxide absorbs the light. This picture suggests how the light from a light bulb differs from sunlight. Actually a light bulb emits even less visible light than this picture shows. 1 Light bulbs do not emit very much blue light, and 97% of the light they emit is infrared light. This means that for every $1 you pay for electricity for lighting, you only get 3 cents worth of light, and the rest is infrared light. Clearly, this is not very efficient. A small improvement in efficiency can be achieved by putting a gas in the bulb that contains chlorine. Light bulbs burn out because the tungsten evaporates from the filament. The chlorine in the bulb captures the tungsten atoms Tungsten + chlorine <=>tungsten chloride gas When the tungsten chloride gas touches the filament, it breaks up into tungsten + chlorine, which repairs the filament. The best part is that the most atoms are delivered to the hottest part of the filament, which is the thinnest part that is about to burn out. The result is that the light bulb can be run a little hotter, so that the light is bluer and the bulb is a little more efficient. If you go to a lighting store, most of the lamps will be designed for incandescent lights. But this is an obsolescent technology. You will not find very many incandescent light bulbs in commercial lighting, because other kinds of lighting are less expensive to operate. Observing the spectrum A diffraction grating has a pattern of very fine grooves on it – 10000 to the inch! It deflects some of the light into a new direction, that depends on the frequency of the light. Low frequency (red) light is deflected more than high frequency (blue). When you view a light source through a diffraction grating, you see a "rainbow" (physicists call this a spectrum). Incandescent lights make all frequencies of light, so we get a complete spectrum with no features. As we will see, other light sources make more interesting spectra. The light source has to be small to give a good spectrum. When an object is not small, each part of it makes a spectrum, and these overlap. For example, here is a light source that is making red light, yellow light, green light, blue light, and ultraviolet light. We see 5 overlapping copies of the light source instead of a spectrum. For this reason, a light source is sometimes viewed through a slit, to make it look like a small light. 2 The color of objects When light strikes an object, each kind of light is either absorbed or reflected. A red object is one that reflects red light and absorbs blue and green; a blue object is one that reflects blue (and at least partially absorbs red and green). The eye isn’t very sensitive to details of the spectrum. All colors that we can perceive can be imitated by some combination of red, green, and blue light. The color yellow can be simulated by many combinations of red and green light, as well as by light that happens to have a particular frequency. Yellow objects usually are absorbing blue and reflecting as much red and green (and everything in between), which is why yellow tends to be a bright color. Einstein's other equation Einstein got the Nobel Prize for discovering that light delivers its energy in lumps, like bullets, instead of a continuous stream of energy. The “bullets” are called photons. The energy of a photon is proportional to the frequency Energy = constant* frequency The constant = 6.6 * 10^-34 Joule-sec., so the energy delivered by one photon is pretty tiny compared to the energy scale we can detect. To a physicist, radio waves, microwaves, Xrays, and gamma rays (one of the forms of radiation from nuclear decay) are all electromagnetic waves, and thus physically similar to visible light. They are related as indicated in this diagram: Note that the scale is in Hertz, which is the unit for measuring frequency. So the scale is also a scale of energy, but arranged in powers of 10 – so a visible-light photon is a million times more energetic than a microwave photon, and a thousand times less energetic than an Xray. 3 A light beam delivers a very large number of photons every second, so that we don't notice the lumpiness. But at the atomic scale it is important; Einstein's equation explains why Xrays and UV light can cause chemical reactions (like sunburn): the photons have enough energy to break chemical bonds. This is also why we can't see infrared light: an infrared photon doesn't have enough energy to make the necessary chemical reaction happen (we can't see ultraviolet light because the stuff in our eye absorbs it before it gets to the retina, to prevent getting a sunburn there). Atoms can only have certain energies; these “states” are well separated. When an atom changes state, the energy is usually emitted as one photon, whose frequency is determined by Einstein’s equation. The result is a spectrum with only a few frequencies represented. When the source is viewed through a slit, the spectrum consists of a set of “lines” (which actually are images of the slit). Here is the spectrum of mercury vapor with an electric current running through it: The source is right at the left edge of this picture (we are looking at the light through a slit). The spectrum consists of just four frequencies: yellow, green, blue-green, and ultraviolet (the camera can see the ultraviolet line, even though our eyes cannot). The mercury vapor light is rather efficient, and so it is sometimes used for outdoor lighting in a parking lot. The light looks a bit blue, and because there is no red light in it at all, red objects look brown because there is no red light to reflect. This color effect is why it is not used very much. There are street lights that have something added to the vapor that emits red light, and make red things look better. 4 Fluorescent lighting The mercury vapor light is the basis for fluorescent lighting. There are materials called phosphors that absorb ultraviolet light and then emit other frequencies. There are two kinds of fluorescent lights in use The top spectrum is the mercury spectrum. The second is the older kind of fluorescent light. The green, yellow, and blue lines are due to the mercury, and the rest of the spectrum is white light provided by the phosphor. The bottom picture is a new kind of fluorescent light (the swirly light bulbs use this phosphor, and the tube-shaped fluorescent lights frequently are using it too – but ten years ago, the tubes all the halophosphor spectrum). Turning the ultraviolet light into visible light lowers the efficiency: we are trading in a high energy ultraviolet photon for a lower energy photon of lower frequency. However, they are still significantly more efficient than incandescent lights. Street lights and other outdoor lighting frequently use a modified mercury vapor light. The picture below shows how the simple spectrum is turned into something that contains many more frequencies. This is the mercury spectrum Putting other metals in with the mercury give a more complex spectrum. The third panel is a street light. The bottom panel is a very common kind of outdoor lighting – all the lights in a stadium or a grocery store parking lot are of this kind. This is also the kind of light used in upscale cars (the ones with the "blue" headlights). 5 Still another kind of light source is in the orange-colored street lights. These are driving an electrical current though high pressure sodium gas. They have a rather complicated spectrum. Light emitting diodes In a semiconductor, the electrons have a definite energy, but this is different for different semiconductors. Light emitting diodes have a junction where different semiconductors meet. When electrons move from one material to another, the energy changes. As each electron moves across the boundary, it changes energy and emits light. The frequemcy of the light is determined by the change in energy of the electron, according to Einstein's equation. The red dots represent the electrons, which are moving to the right. As each electron falls off of the energy cliff, it emits a photon. This is a very efficient process, but makes light of just one frequency. Sometimes this is good: the red, yellow, and green parts of a stoplight make use of three different kinds of light emitting diode. Stoplights used to have an incandescent light bulb and colored glass; the colored glass absorbed the light of the wrong color. This was very inefficient; light emitting diodes are much better (and they last longer, too). There are two ways to make white light with light emitting diodes: put diodes of all the different colors together, or use a light emitting diode that makes ultraviolet light and a phosphor to convert it into visible light. The latter kind of light source is now on sale, but they are somewhat expensive. Since this kind of light source lasts a long time and is inexpensive to operate, it may become the most common kind of light source. 6 Efficiency of the various lighting types This table compares the efficiency of the different kinds of light source. A "lumen" is a measure of brightness of visible light; the two columns give the same information in different units. Type Lumens/watt efficiency Candle 0.3 0.2% Incandescent 16 2.6% Halogen 20 3.2% Fluorescent 60 9% Compact Fluorescent 80 11% Light Emitting Diode 90 13% Metal Halide 100 15% High pressure sodium 180 20% The value for light emitting diodes is for the currently available types. Potentially they will get much better in a few years. Already they give 5 times more light than from an incandescent light bulb. Here is an example to illustrate the relevance of the comparison. A light bulb lasts about 1000 hours. The cost of electricity is about $0.10 per kilowatt hour. In 1,000 hours, a 100 W incandescent light bulb will use 100 kwhr at a cost of $10; a compact fluorescent light bulb will only use a fifth of this, and only cost you $2.00. This table describes 4 light bulbs bought at a store in Lexington on March 27, 2011. Type Price Lifetime Watts input Light output Incandescent $3.76 3000 H 45 W 330 lumens Halogen incandescent $6.26 3000 H 45 W 490 lumens Compact fluorescent $7.48 10,000 H 11 W 400 lumens Light emitting diode $26.98 50,000 H 8W 350 lumens Just on the basis of the expected lifetime, the light emitting diode is hardly more expensive than an ordinary light bulb; the energy savings makes it a big winner. 7