Exam2 outline
advertisement

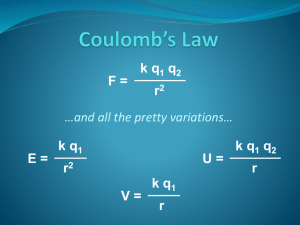
Solution Equilibrium exam outline Solutions: Concentration units: molal (m) moles/kg solvent %(mass) g/g solution ppm g/106g total or μg/g total ppb g/109g total or ng/g total mole fraction(X) moles/moles total molarity (M) moles/L solution Raoult’s Law Ptot = PA + PB = XAPºA +XBPºB Ideal solutions: no intermolecular forces. Pressure just the sum of individual pressures times the fraction of molecules of that type. Freezing point depression For nonvolatile solutes: ∆Tf = kf i m ∆Tb = kb i m k’s property of solvents i = # ions / molecule Remember Tf = Tºf - ∆Tf Tb = Tºb + ∆Tb Calculate new freezing or boiling temperature given solute and concentration or the i factor given concentration and Tf. Change of vapor pressure with temperature: H vap P ln 1 R P2 1 1 T1 T2 Calculate ∆H given P,T data or Calculate new P or T given ∆H and P,T data. Osmosis Π = M RT Osmotic pressure (Π ) increases with molarity of solute. More concentrated side absorbs solvent, rises in U tube. Concepts: Intermolecular forces (see solutions HW) Ideal Solutions, what are the assumptions Solubility and temperature Enthalpy of solution Equilibrium: Know how to write the mass action expression for the equilibrium relation for a balanced reaction. [ A]a [ B]b K [C ]c [ D]d aA + bB --> cC + dD Solids and pure liquids not included in rate expression. LeChatellier’s Principle and direction of reaction. Calculate K from equilibrium concentrations: Given [A], [B], [C], [D] plug these into the expression for K above and calculate the value for K. Relate Kc to Kp using P = (n/V) RT = [ ] RT So Kp = Kc (RT)(nprod-nreact) Calculate the equilibrium concentrations given K and the beginning concentrations. You can shift concentrations to product or reactant side and not affect the equilibrium values. DON’T FORGET THE STOICHIOMETRY! Examples of approximations when K is large or small: Small K: HCN (aq) H+(aq) + CN-(aq) K = 4.9 x10-10 Init.: 0 5x10-3 0.001 Mod. Init .001 4x10-3 0 Change -x +x +x Equilib .001-x 4x10-3+x K tiny, shift to reactant side x K small, so x<<.001, drop from first two entries above since it is insignificant compared to those. So K Giving [CN ][ H ] x(4 103 ) 4.9 1010 4x [ HCN ] .001 [HCN] = .001M [H+] = 4x10-3 M x 1.2 1010 [CN-]= 1.2x10-10M Large K: Cu+ Init.: + .006 2CN- .015 Cu(CN)20 Mod. Init 0 3x10-3 .006 Change +x +x -x Equilib x 3x10-3+x K=1.0 x 1016 K large, shift to product side .006 - x K large, so x<<.001, drop from last two entries above since it is insignificant compared to those. Approx: So, K x 3x10-3 .006 [Cu (CN )2 ] .006 1.0 1016 2 [Cu ][CN ] x(.003)2 Giving [Cu+] = 6.7 x 10-14 M so x 6.7 1014 [CN-] = 3x10-3 M [Cu(CN)2-] = .006 M Suppose on the first example you do not shift to the reactant side. HCN (aq) H+(aq) Init.: 0 Change +x Equilib x CN-(aq) + 5x10-3 K = 4.9 x10-10 0.001 -x K tiny, shift to reactant side -x 5x10-3-x .001-x [CN ][ H ] (.001 x)(5 103 x) 5 106 6 103 x x 2 10 K 4.9 10 [ HCN ] x x 4.9 1010 x 5 106 6 103 x x 2 x 2 (6 103 4.9 1010 ) x 5 106 0 Drop the 4.9 x10-10 added to 6x10-3 : x 2 6 103 x 5 106 0 (.001 x)(.005 x) from above. So, either from quadratic equation or looking back at the original equilibrium equation factors, x = .001 or .005, the second is unphysical since it leaves [CN-] negative. This gives: [HCN] = .001M [H+] = 4x10-3M [CN-] = 0 Oops! Just put the nonzero concentrations into the equilibrium expression and put in a variable, y, for the zero concentrations: 4.9 1010 (.004) y 4 y y 1.2 1010 .001 [HCN] = .001M giving [H+] = 4x10-3M [CN-] = 1.2x10-10 M So you can recover from this if you forget to approximate. More examples on the links page. Energy and Equilibrium: ΔG =ΔH – TΔS = -RTln(Q) Q = K expression with actual concentrations substituted. If ΔG <0 for a reaction, it is spontaneous, will proceed forward If ΔG = 0 reaction is at equilibrium, concentrations won’t change If ΔG >0 Reaction will proceed toward reactants, non-spontaneous.