u14q equilibrium diagnostic KEY
advertisement
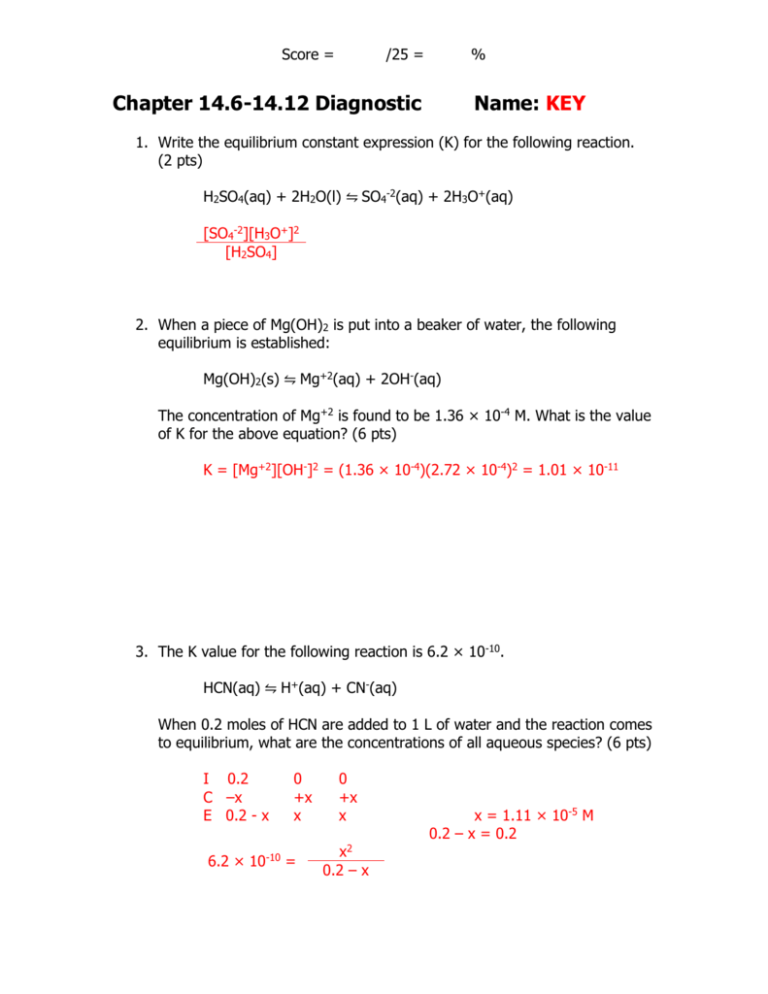
Score = /25 = % Chapter 14.6-14.12 Diagnostic Name: KEY 1. Write the equilibrium constant expression (K) for the following reaction. (2 pts) H2SO4(aq) + 2H2O(l) ⇋ SO4-2(aq) + 2H3O+(aq) [SO4-2][H3O+]2 [H2SO4] 2. When a piece of Mg(OH)2 is put into a beaker of water, the following equilibrium is established: Mg(OH)2(s) ⇋ Mg+2(aq) + 2OH-(aq) The concentration of Mg+2 is found to be 1.36 × 10-4 M. What is the value of K for the above equation? (6 pts) K = [Mg+2][OH-]2 = (1.36 × 10-4)(2.72 × 10-4)2 = 1.01 × 10-11 3. The K value for the following reaction is 6.2 × 10-10. HCN(aq) ⇋ H+(aq) + CN-(aq) When 0.2 moles of HCN are added to 1 L of water and the reaction comes to equilibrium, what are the concentrations of all aqueous species? (6 pts) I 0.2 C –x E 0.2 - x 0 +x x 6.2 × 10-10 = 0 +x x x2 0.2 – x x = 1.11 × 10-5 M 0.2 – x = 0.2 4. The K value for the following equation is 240. Pb+2(aq) + 3Cl-(aq) ⇋ PbCl3-(aq) A solution contains the following concentrations of ions: Pb+2: 0.2 M Cl-: 0.1 M PbCl3-: 0.05 M Which way will the reaction go to reach equilibrium? Show your work. (6 pts) Q= 0.05 = 250 (0.2)(0.1)3 Q > K, so reaction will go left. 5. The following reaction is exothermic. A(aq) + B(s) ⇋ C(aq) + D(g) Predict which way the reaction will shift when the following things occur: (5 pts) a. Some A is removed from solution. left b. Some B is added to the solution. no change c. Some C is removed from the solution. right d. The reaction vessel is cooled down. right e. The volume of the vessel is decreased. left