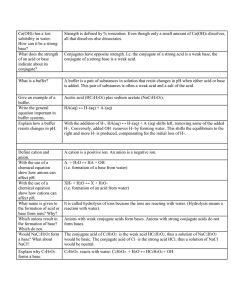
Varied Equil/Acid/Base
advertisement

NAME ________________________________________ RULES: For any credit you must answer every question, in the event of multiple questions. No part(s) may be skipped. You should print out this page, and show your work and your answer(s) on this page. Pencil/Pen/Type are each acceptable. You must turn in this page, by the end of the school day (2:10 p.m.) on which it is due. Partial credit is assigned only under particular circumstances; upon which I decide. (e.g. double jeopardy) The earned points will be applied to your overall point score, as bonus. The work must be absolutely legible to me. If I must guess as to meaning, I shall always guess, incorrectly. You are welcome to use your text, Trivedi flash, internet sources, a friend’s understandings for research purposes. All work however, must be your own. Extended responses must reflect your understanding of the issue …hence you must work in good faith. AP BONUS 4: EQUILIBRIUM/ACID/BASE/SALTS DUE: 8 JANUARY 2016 PLEASE READ: Many of the following deal with directly or peripherally with equilibrium and/or acid/base theory. You will be asked to; manipulate equilibria, use stoichiometry, use molarity …etc. Thus you are asked to employ and/or integrate prior learning and knowledge of chemistry…. Because of the number of questions, each is worth only 0.5 point…yielding a perfect score of 4 bonus points. 1) What is the equilibrium constant for the following reaction? N3-1 + H3O+1 ↔ HN3 + H2O The Ka value for hydrzazoic acid, HN3, = 1.9 x 10-5. Show your work. 1) 2) 3) 4) 5) 5.3 x 10-10 1.9 x 10-9 1.9 x 10-5 5.3 x 104 1.9 x 109 2) The pH of 0.1-molar ammonia, (a weak base) is approximately… 1) 1 2) 4 3) 7 4) 11 5) 14 3) 3 Ag(s) + 4 HNO3 3 AgNO3 + NO(g) + 2 H2O The reaction of silver metal and dilute nitric acid proceeds according to the equation above. When 0.10 mole of powdered silver is added to 10. milliliters of 6.0-molar nitric acid, the number of moles of NO gas that can be formed is ________. Show your work. 1) 2) 3) 4) 5) 0.015 mole 0.020 mole 0.030 mole 0.045 mole 0.090 mole 4) When 70. milliliter of 3.0-molar Na2CO3 is added to 30. milliliters of 1.0-molar NaHCO3 the resulting concentration of Na+ is ________. Show your work. 1) 2) 3) 4) 5) 2.0 M 2.4 M 4.0 M 4.5 M 7.0 M 5) Equal volumes of 0.10-molar H3PO4 and 0.20-molar KOH are mixed. After equilibrium is established, the type of ion in solution in largest concentration, other than the K+ ion, is 1) 2) 3) 4) 5) H2PO4¯ HPO42¯ PO43¯ OH¯ H3O+ 6) The reaction shown here has an equilibrium constant equal to 3.7 x 104. Which of the following can be concluded from this information? HC2H3O2 + CN- ↔ HCN + C2H3O2− 1) 2) 3) 4) 5) CN- is a stronger base than C2H3O2− HCN is a stronger acid than HC2H3O2 The conjugate base of CN- is C2H3O2− The equilibrium constant will increase with an increase in temperature. The pH of a solution containing equimolar amounts of CN- and HC2H3O2 is 7.0. 7) Calculate the [H+] in a solution that has a pH of 11.75. Show your work. 1) 2.3 M 2) 11.7 M 3) 5.0 × 10–3 M 4) 1.8 × 10–12 M 5) none of these 8) Which of the species below, when dissolved in H2O, will NOT produce a basic solution? Defend your Thinking (just simply…). 1) SO2 ________________________________________________________________________ 2) NH3 3) BaO ________________________________________________________________________ 4) Ba(OH)2 5) none of these ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________