CaCO3 Expt V2
advertisement

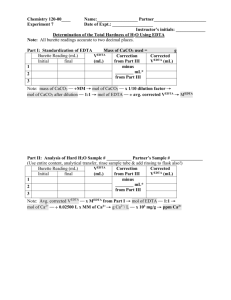
CE 420/520 EXPERIMENT - CALCIUM CARBONATE EQUILIBRIA (adapted from Water Chemistry Lab Manual, 3 rd edition) Due date: Oct. 28, 2004 1. Purpose To determine whether certain unknown solutions are at equilibrium with respect to calcium carbonate precipitation. 2. Theory The calcium carbonate system is important in both natural waters and in water treatment systems. Treated waters, for example, frequently require adjustment prior to passage through sand filters or before discharge to water distribution mains because the calcium carbonate precipitation reaction does not reach equilibrium in the contact time allowed in the water treatment plant. Care must be exercised in adjusting a supersaturated solution to prevent precipitation because if an excessive adjustment is made, the water will become aggressive and will tend to dissolve calcium carbonate which in some systems forms a protective layer inside water mains. The equation for dissolution of calcium carbonate is CaCO3(s) < === > Ca2+ + CO32- Kso = 10 -8.34 (1) And the equations for protonation of carbonate ion are: CO32- + H+ < == > HCO3HCO3- + H+ < == > H2CO3* K = 10+10.33 K = 10 +6.35 (2) (3) Since many natural water systems have pH values in the range where the bicarbonate is the predominant species a more useful equation is obtained by adding (1) and (2). CaCO3(s) + H+ < === > Ca2+ + HCO3- K = 10+1.99 (4) Various procedures exist for determining if a system is at equilibrium with respect to calcium carbonate. One involves calculating the free energy of the system. The free energy change, G is zero if the reaction is at equilibrium; i.e., Q = K. This is given by the following equation: G = G0 + RT ln Q = - RT ln K + RT ln Q HCO 3 a Ca 2 a G = RT ln (5) H a K If [HCO3-], Ca2+ and [H+] are analyzed in the water sample, the analytical values can be substituted in the above equation to determine whether equilibrium exists. An alternative and equivalent procedure is to calculate the Langelier Index, LI, for the system : LI = pHa - pHs where pHa is the analytical pH and pHs is the saturation pH. pHs is calculated by inserting measured concentrations of HCO3- and Ca2+ in the expression for the equilibrium constant for equation 4 K= HCO Ca ( [H 3 2 HCO3 HCO Ca if ionic strength is impor tan t) [ H 3 H Ca 2 2 (6) and solved for [H+]. The [H+] concentration when computed by using this procedure is the concentration that would exist if the solution were at equilibrium with calcium carbonate. The negative log of this value is pHs. If LI > 0, the solution is supersaturated with respect to CaCO3(s), and if LI < 0 dissolution of CaCO3(s) tends to occur. If LI = 0, the solution is in equilibrium with CaCO3(s). 3. Procedure 1. The following solutions to be prepared a. A CaCO3 solution prepared 3 days ago b A CaCO3 solution prepared 1 days ago 1 c. d. e f. 2. 3. 4. 5. A CaCO3 solution prepared 2 hours ago A CaCO3 solution prepared 0.5 hours ago Acidified distilled water sample that has been in contact with solid CaCO3(s) for approx. 1 day. Tap water Measure the pH of all samples before filtration using a carefully standardized pH meter. If there are visible amounts of suspended CaCO3(s) particles present, filter samples of solutions "a" through "e" through fine filter paper. Measure Ca2+ in all samples by EDTA procedure. Determine alkalinity (Total, carbonate and caustic) of all samples by titration with H2SO4. Use unfiltered samples but make certain that the sampling procedure does not permit CaCO3(s) to become suspended. (If CO2 escapes or is absorbed before the alkalinity titration, the carbonate alkalinity but not the total alkalinity will be affected. 4. Lab report and Data Analysis The lab report should include a brief introduction, objectives, methods and materials, results and discussion. Answer the following questions. 1. 2. 3. 4. Calculate whether each solution is at equilibrium with CaCO3(s) or whether precipitation or dissolution of calcium carbonate is tending to occur. For samples "a" through "e" plot [Ca2+], [HCO3-]a and LI vs time. Include the values of each these quantities at time zero. (Note [HCO3-] = 1CT, CO3 . At pH 8.3, 1 = 1 but at pH 9.5, 1< 1. See text book, Appendix 1 for values). From these data what can you deduce about the rate of CaCO3(s) precipitation in this system? What implications does this have for water treatment processes? If the LI does not reach zero after 3 days, will it reach zero if the constants are corrected for temperature and if ionic strength effects are taken into account (see text book) for procedures)? Estimate the ionic strength using the measured alkalinity and calcium values. For CE 520 students only There are many other saturation/stability indices. Provide descriptions and the merits of the following two: Ryznar Stability Index (RSI) and the Puckorius Scaling Index (PSI). If possible compute the RSI and PSI of the waters tested above. Compare and comment on your results with the LI. 5. Apparatus Burettes and pipettes Filter paper and filter funnels Magnetic stirrer Chemical Reagents (see below) Volumetric flasks and beakers pH meter Indicators 6. Reagents a. Calcium carbonate solution – sample solutions Prepare a supersaturated CaCO3 solution by mixing equal volumes of an 8 x 10-3 M sodium carbonate solution adjusted to pH 9.5 with NaOH and a 4 x 10 -3 M Calcium chloride solution similarly adjusted to pH 9.5. Prepare individual batches of this solution at the following times prior to the experiments: 3 days, 1 day, 2 hour, 0.5 hours prior to the experiments. Stir each solution continuously keeping contact with the atmosphere to a minimum. (Approximately 500 mL of each solution per student group). b. One day in advance of the laboratory period adjust the pH of distilled water to 3.0. Add sufficient solid CaCO3(s) so that solid remains after one day. Do not readjust pH. (Approximately 500 mL per group). c. Standard EDTA solution : 0.01 M Standard Na2CO3 solution: 0.02 N Standard H2SO4 solution: 0.02N Hydroynaphtanol blue indicator or equivalent. For cation determination pH buffers for standardizing pH meter: pH 4. 7 and 10 NaOH: 1 M Bottle with dropper : 50 mL per group Phenolphthalein and methyl orange indicator 2 7. Standardization and Titration Procedures pH meter - standardize the pH meter using the buffer solutions Alkalinity titration 1. If the sulfuric acid is not standardized, - prepare 1 liter of standard N Na2CO3 by dissolving 1.060 g of anhydrous reagent grade Na2CO3 (dried at 140o C for 24 hours in distilled water) - standardize the sulfuric acid (0.02 N) by titrating three 50 mL of standard Na 2CO3 solution with the acid using the pH meter and titrating to pH 4.3. 2. If the sulfuric acid is already standardized, titrate 100 mL volume (V) of each sample in 250 mL conical flask as follows: - measure the initial pH of the solution with a pH meter - add 4 drops of brom cresol green indicator solution and titrate with 0.02 N sulfuric acid. The end point is when the blue color completely disappears and the solution is yellow. Calculation: Total Alkalinity expressed as mg/L CaCO3 = Vol of acid (mL) x Normality of Acid x 50,000/ Vol. of Sample (mL) Note that initial pH of the solution will indicate whether the predominant alkalinity species is the bicarbonate species. If this is so then alkalinity is equal to [HCO3-]. To use equations (5) or (6), concentrations are in moles/liter. Calcium Determination Principle: Chelating reactions between divalent cations (example, Ca2+) and ethylene diamine tetracetic acid (EDTA) are typically used in the determination of divalent cations (Ca2+, Mg2+) and hardness. An indicator such as hydroxynaphthol Blue (HNB) is added to form a red complex with Ca2+. When EDTA is added to the HNB- Ca2+ complex, Ca2+ is withdrawn into an EDTA- Ca2+ chelate and the color of the solution changes from the red color of the HNB- Ca2+ complex to the blue color of free HNB: HNB (blue) + Ca2+ < == > HNB Ca2+ (red) The titration is conducted at pH > 12 so that Mg2+ precipitates as Mg(OH)2(s) and does not react with the EDTA during the titration of Ca2+. At this pH, CaCO3 (s) also may precipitate and subsequently during the titration slowly re-dissolve to cause a fading endpoint. This difficulty can be overcome by performing a trial titration to determine the approximate endpoint. The exact titration is performed by adding almost all of the required titrant before adjusting the pH > 12 then rapidly finishing the titration. 1. 2. 3. 4. 7. Measure 50 mL sample into a 125 mL Erlenmeyer flask Add 3 mL of 1 N sodium hydroxide. Measure pH with a pH meter. If less than 12, add NaOH until the pH is 12 or higher. Add 1 scoop (approx. 0.1 g) of hydroxynaphthol blue indicator powder. Do a preliminary titration with 0.01 M EDTA until the color changes from red to royal blue. Repeat titration as above but omit the addition of NaOH and indicator until prior the approximate endpoint indicated by the preliminary titration. Then rapidly titrate but carefully complete the titration by observing the disappearance of red color and the first appearance of pure blue. It is normal for the color change to reverse on standing – there is no need to titrate further. Calculation: Calcium hardness expressed as mg/L CaCO3 = (mL EDTA) x (0.01 M) x (100 g/mole CaCO3 ) x (1000 mg/g) / (mL of Sample) Calcium (moles/L) = (mL EDTA) x (0.01 M) / (mL of Sample) 3