exam-1a - Prince George`s Community College
advertisement
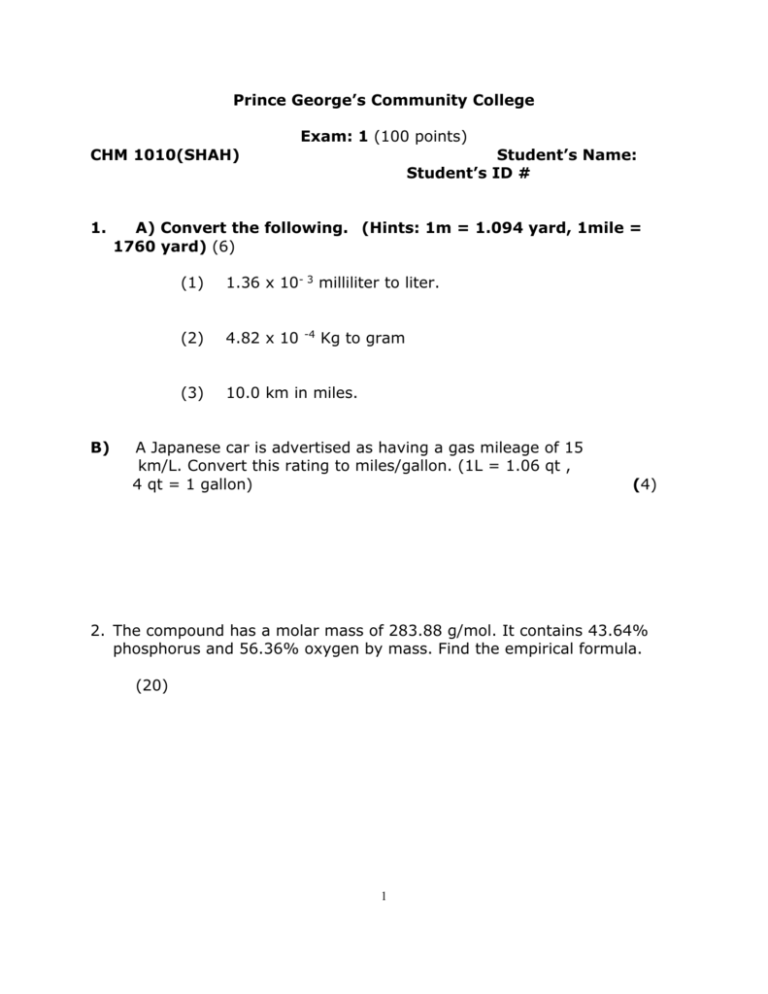
Prince George’s Community College Exam: 1 (100 points) CHM 1010(SHAH) 1. B) Student’s Name: Student’s ID # A) Convert the following. (Hints: 1m = 1.094 yard, 1mile = 1760 yard) (6) (1) 1.36 x 10- 3 milliliter to liter. (2) 4.82 x 10 (3) 10.0 km in miles. -4 Kg to gram A Japanese car is advertised as having a gas mileage of 15 km/L. Convert this rating to miles/gallon. (1L = 1.06 qt , 4 qt = 1 gallon) (4) 2. The compound has a molar mass of 283.88 g/mol. It contains 43.64% phosphorus and 56.36% oxygen by mass. Find the empirical formula. (20) 1 3. Calculate the molar mass of calcium carbonate (CaCO3). A certain sample of calcium carbonate contains 4.86 moles. What is the mass of the CO32- ions present ? (20) 4. Balance the equation if necessary and identify the type of chemical reaction. (10) (A) NH3 + O2 = NO + (B) CaCO3 = CaO + CO2 (C) Mg + O2 = MgO H2O (D) KOH + H2SO4 = K2SO4 + H2O (E) Ca(OH)2 +HCl = CaCl2 + H2O 5. Al + O2 = Al2O3 balance the equation (20) If 8.1 g of Al react with O2 how many moles of O2 are required ? How many gram of Al2O3 will be produced ? 2 6. Give the IUPAC name for the following compounds. (10) (a) CaO (b) AgNO3 (c) Ca(OH)2 (d) NaNO3 (e) FeCl3 (f) NaCl (g) Al2O3 (h) KMnO4 (i) KCl (j) CuSO4 7. Give the molecular formula for the following compounds. (10) (a) Methanol (Methyl alcohol) (b) Hydrogen Peroxide (c) Sulfuric acid (d) Sodium Carbonate (e) Ammonium Chloride (f) Sodium bicarbonate 3 (g) Cobalt II Chloride (h) Potassium Nitrate (i) Hydrochloric acid (j) Methane 4











