Alkalinity Determination: Lab Experiment Guide
advertisement
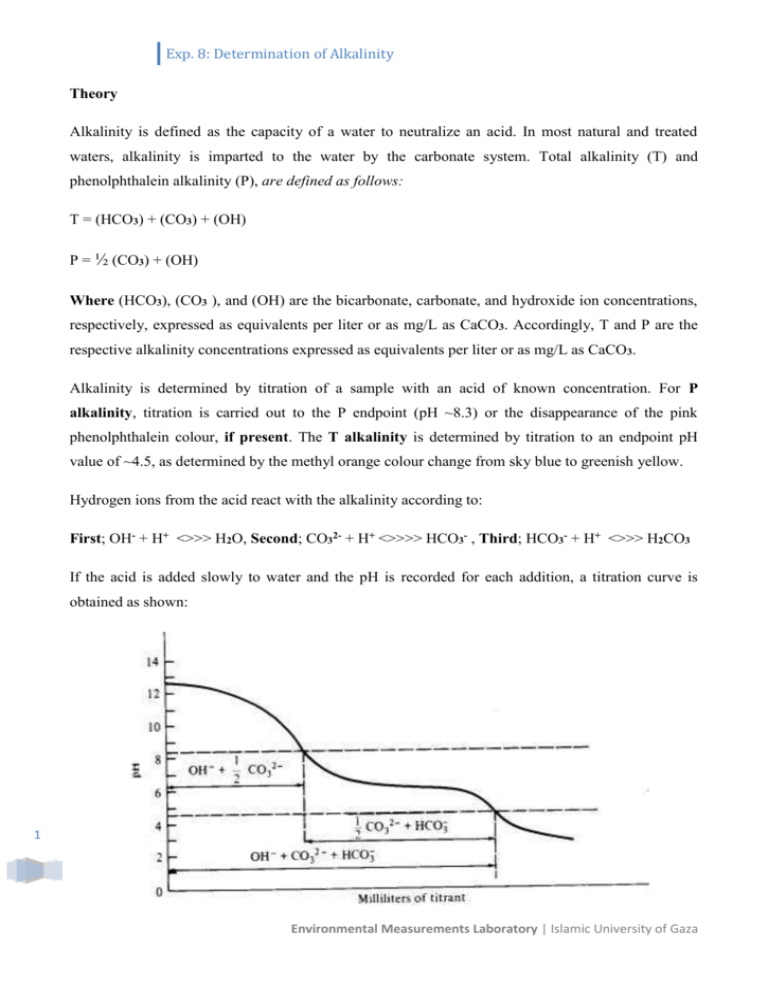
Exp. 8: Determination of Alkalinity Theory Alkalinity is defined as the capacity of a water to neutralize an acid. In most natural and treated waters, alkalinity is imparted to the water by the carbonate system. Total alkalinity (T) and phenolphthalein alkalinity (P), are defined as follows: T = (HCO3) + (CO3) + (OH) P = ½ (CO3) + (OH) Where (HCO3), (CO3 ), and (OH) are the bicarbonate, carbonate, and hydroxide ion concentrations, respectively, expressed as equivalents per liter or as mg/L as CaCO3. Accordingly, T and P are the respective alkalinity concentrations expressed as equivalents per liter or as mg/L as CaCO3. Alkalinity is determined by titration of a sample with an acid of known concentration. For P alkalinity, titration is carried out to the P endpoint (pH ~8.3) or the disappearance of the pink phenolphthalein colour, if present. The T alkalinity is determined by titration to an endpoint pH value of ~4.5, as determined by the methyl orange colour change from sky blue to greenish yellow. Hydrogen ions from the acid react with the alkalinity according to: First; OH- + H+ <>>> H2O, Second; CO32- + H+ <>>>> HCO3- , Third; HCO3- + H+ <>>> H2CO3 If the acid is added slowly to water and the pH is recorded for each addition, a titration curve is obtained as shown: 1 Environmental Measurements Laboratory | Islamic University of Gaza Exp. 8: Determination of Alkalinity P is the amount of acid required to reach pH 8.3 , M is the total quantity of acid required to reached pH 4.5, The following generalizations can be made: Result of titration Hydroxide (OH-) Carbonate (CO32-) Bicarbonate (HCO3-) P=0 0 0 T 2P < T 0 2P T – 2P 2P = T 0 2P 0 2P > T 2P – T 2 (T - P) 0 P=T P 0 0 Reagents 1) HCl (0.1 N) 2) Phenolphthalein indicator solution ( pH 8.3 indicator ), and 3) Bromo Cresol green indicator. Procedure 1) Measure 50 mL of each sample. (Use graduated cylinder). 2) Fill burette with standardized HCl 3) Add 2-4 drops of phenolphthalein indicator to the sample and titrate to endpoint with HCl; record the mL required. This is P-alkalinity. 4) Add 2-4 drops of Bromo Cresol green indicator to the same sample, and titrate to pH 4.5 endpoint or to greenish yellow colour. This is T-alkalinity. 5) Calculate P and T-alkalinity in mg/L as CaCO3. 6) Calculate hydroxide, carbonate and bicarbonate concentrations. Calculation: 2 From the titration results, the concentrations (as equivalents/L or mg/L as CaCO3) of the different forms of alkalinity may be calculated as follows: Environmental Measurements Laboratory | Islamic University of Gaza