Quantitative RT-PCR (MSWord doc)
advertisement
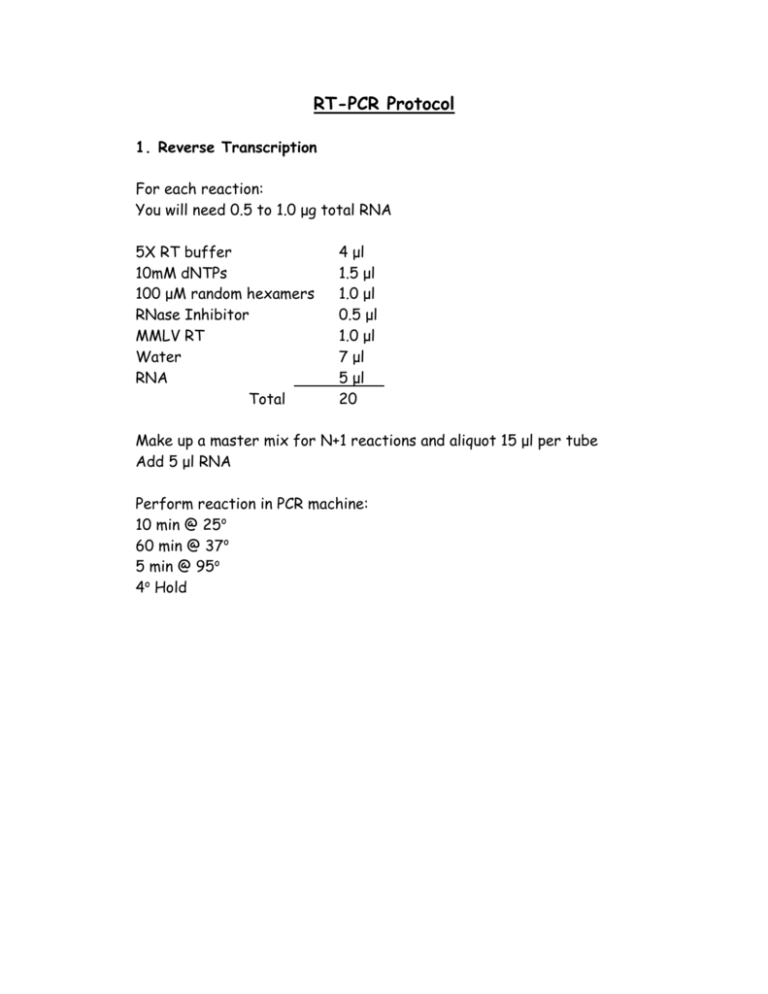
RT-PCR Protocol 1. Reverse Transcription For each reaction: You will need 0.5 to 1.0 μg total RNA 5X RT buffer 10mM dNTPs 100 μM random hexamers RNase Inhibitor MMLV RT Water RNA Total 4 μl 1.5 μl 1.0 μl 0.5 μl 1.0 μl 7 μl 5 μl 20 Make up a master mix for N+1 reactions and aliquot 15 μl per tube Add 5 μl RNA Perform reaction in PCR machine: 10 min @ 25o 60 min @ 37o 5 min @ 95o 4o Hold 2. QUANTITATIVE PCR Titanium Taq is from Clontech We use Titanium Taq for everything except human GAPDH (for some odd reason the human GAPDH primers only work in the Platinum Taq buffer) All reactions are done in duplicate. 1. Thaw all reagents at room temperature and then keep on ice. Thaw cDNA samples and place on ice. cDNA samples can be usually be diluted 1:4 with TE. 2. For each gene, generate a Master Mix which contains everything except the cDNA sample. Keep on ice. 3. Add 2 µl cDNA sample to each well of the PCR plate or strip. 4. Aliquot 23 µl of Master Mix into each well of the PCR plate or strip. 5. Cover and quick spin. 6. Put into Opticon and start the appropriate program. For most of the primers that we have developed the following conditions work well: QUANTITATIVE PCR WORKSHEET Titanium Taq Primer pairs : ________________________ cDNA source :________________________ Amount of cDNA added :___________ Program:_____________________________ Number of Samples :_____ N Reactions= Number of samples + 1 1X Water 10X PCR Buffer 50 mM MgCl2 10 mM dNTPs 10 µM Primer (F) 10 µM Primer (R) 100X SYBR green Titanium Taq Total 16.45 2.5 0.5 1.0 1.0 1.0 0.25 0.3 23 For N reactions Other Notes: 1. Calculations: Everything is normalized to the expression of HPRT so the first gene we always do PCR for is HPRT. Once you have that data, you can go back at anytime to your RT reaction and test your gene of interest. Gene expression levels are expressed as relative mRNA units according to the formula 2(HPRT C(T) – Target C(T)) C(T)= Average threshold cycle of the duplicate reactions as determined using the Opticon Monitor software. 2. Reagents and sources: M-MLV reverse transcriptase: Invitrogen # 28025-013 RNase Inhibitor: Invitrogen # 15518-012 Random hexamers: Invitrogen # 48190-011 Titanium taq: BD Biosciences (Clontech) # 639209 Sybr Green: Molecular Probes # S7563 3. Primer design We design our primers so that they are in separate exons using the Primer3 program http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi Tm for the oligos are set to be between 64o and 68o with a product length of 100-200 bps. Optimal size of primers is 20 bases. 4. Reference: A good source for info on quantitative PCR is from the ABI 7700 User Bulletin #2.











