Urinalysis Microscopy Procedure-SOP
advertisement

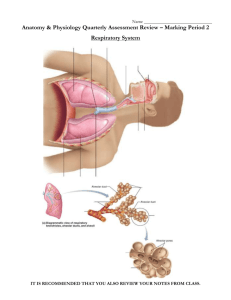
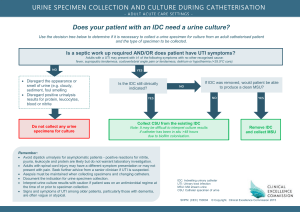
URINALYSIS – MICROSCOPIC EXAMINATION Document Number: Pro68-11 Effective (or Post) Date: 20 Nov 2008 Document Origin Company: MU-JHU Lab SMILE Approved by: Jo Shim Review by Heidi Hanes Review date 7 Feb 2012 SMILE Comments: This document is provided as an example only. It must be revised to accurately reflect your lab’s specific processes and/or specific protocol requirements. Users are directed to countercheck facts when considering their use in other applications. If you have any questions contact SMILE. Author: Ali Elbireer CORELAB, MU-JHU RESEARCH COLLABORATION URINALYSIS – MICROSCOPIC EXAMINATION Procedure: Urinalysis Microscopic Examination SOP ffective Date: Jun 05, 2005 Revision #2 Supersedes Rev# 1 April 03, 2003 Revised/Prepared By Signature Date Signature Date Signature Date Ali Elbireer Approved By Laboratory Medical Director Laboratory Administrator Annual Review By (Lab Supervisor/Lab Management) 05 Jun 05 Rev 2.0 Page 2 of 5 CORELAB, MU-JHU RESEARCH COLLABORATION URINALYSIS – MICROSCOPIC EXAMINATION 1. Principle The microscopic examination is a vital part of the routine urinalysis. It is a valuable diagnostic tool for the detection and evaluation of renal and urinary tract disorders as well as other systemic diseases. Urine Microscopy will only be performed per physicians/primary care-giver request for clinical management or per study protocol requirements. 2. Specimen Collection and Handling 2.1. 2.2. 2.3. 2.4. Patient Preparation: none for random, first-morning or post-prandial urine Specimen Requirements: fresh, cleanly voided urine collected in a clean container Specimen Volume: Optimum 20 mL, minimum 1 mL Specimen Handling: Test as soon as possible after collection. 2.4.1. Optimum conditions: test within 1 hour of collection. 2.4.2. Option: refrigerate at 2-80C immediately and test within 12 hours. 2.4.3. Marginal: test upon receipt, note if specimens are un-refrigerated and >4 hours old. 3. Materials Required 3.1. 3.2. 3.3. 3.4. 3.5. Conical centrifuge tube, 15 mL Microscope slides Microscope cover slips Plastic disposable pipette Urine Controls, Hematronix Level 1 and 2 or equivalent 4. Equipment Required 4.1. Microscope 4.2. Centrifuge, 2000 rpm 5. Reagent Preparation – none 6. Storage and Stability 6.1. Hematronix Controls 6.1.1. Unopened product is stable up to the expiration date printed on the label when kept at 2-80C. Do not freeze. 6.1.2. Opened stability is 2 weeks at either 2-80C or 18 - 300C. 6.1.3. Do not store the control material in direct light. 6.1.4. Discard the controls if turbidity or evidence of microbial contamination is present. 6.1.5. Note: It is normal to observe cellular sedimentation in the bottom of the tube when stored for prolonged period. 7. Calibration – none 8. Quality Control 8.1. 8.2. 8.3. 8.4. 8.5. 8.6. Run two (2) control levels each day of use. Check the lot numbers / expiration dates of the controls are listed on the QC log and are current. Record control values on QC log and check that the results are within the expected range. If the QC results fall within the expected range, continue with patient testing. If the QC results are not within range, repeat and consult with the LS/LSA for further action. Document any QC problems or unexpected results on the Corrective Action Log. 9. Procedure 9.1. Note the color, clarity and volume of urine and record on the urinalysis worksheet. 9.2. The microscopic examination should be performed on a 10-15 ml centrifuged sample. 9.2.1. Mix the sample well and pour 10-15 mL into a 15 mL conical centrifuge tube. 9.2.2. Centrifuge at 2000 rpm for 5 minutes. 9.3. If the volume of specimen is too small to be centrifuged (< 0.5 mL) 9.3.1. Examine the sample directly. 9.3.2. Report the results are from an un-centrifuged urine under comments as <0.5 mL, direct exam” 9.4. If the volume of the specimen is <10 mL but > 0.5 mL, centrifuge the sample and report as “short sample, <10mL” under sample comments. 9.5. Pour off the supernatant fluid and re-suspend the sediment in the urine that drains back down the sides of the tube. 05 Jun 05 Rev 2.0 Page 3 of 5 CORELAB, MU-JHU RESEARCH COLLABORATION URINALYSIS – MICROSCOPIC EXAMINATION 9.6. Flick the bottom of the tube to mix the sediment. 9.7. Using a disposable pipette, place a drop of the sediment on a clean slide and cover with a coverslip. 9.8. Examine immediately using subdued light to provide adequate contrast (partially close the iris diaphragm and adjust the condenser). 9.8.1. If the light is too bright, low refractive index objects such as casts could be missed. 9.9. Use the fine adjustment continuously (adjust up and down) to see the depth of the object(s) as well as other structures that may be on a different focal plan. 9.10. First view the sediment under low power (10x). 9.10.1. Scan the slide and observe for casts, crystals and other elements that may be present. 9.10.2. Switch to high dry power (40x) when necessary to delineate the structures that are seen. 9.10.3. Casts have a tendency to move towards the edge of the coverslip; scan the periphery of the coverslip as well. 9.10.4. Note the type and the average number of casts seen per low power field on the worksheet. 9.10.5. Note the presence and type of crystals (crystals need only be reported as present unless they are very abundant). 9.11. View the sediment under high power (40x) 9.11.1. Estimate the number of red blood cells, white blood cells and epithelial cells per high power field by observing 10-15 fields. 9.12. Note any bacteria or yeast (budding or hyphae) and estimate the amount as follows: 1+ approximately 0-25% of the field 2+ approximately 25-50% of the field 3+ approximately 50-75% of the field 4+ full field 9.13. Note any Trichomonas vaginalis seen and estimate the number per field (0-1, 0-3 etc). 9.14. Note any other organisms or objects seen such as glitter cells or clue cells. 9.15. Record the results obtained on the urinalysis worksheet, microscopic section. 10. Expected Values 10.1. Color: Straw to dark yellow 10.2. Clarity: Clear to hazy. 10.3. Casts: None seen. 10.4. WBC’s: 0-3/hpf 10.5. RBC’s: 0-3/hpf 10.6. Epithelial cells: 0-3/hpf 10.7. Crystals: None Seen 10.8. Organisms (bacteria, yeast, trichomonas): None seen. 10.9. Glitter (WBC covered with bacteria) or Clue (epithelial cell covered with bacteria) cells: None seen. 11. Procedural Notes 11.1. If cleanly voided urine is not collected, the microscopic examination may give erroneous results due to the presence of external organisms/artifacts. 11.2. Specimens left at room temperature will begin decomposing due to presence of bacteria and pH changes. Casts and other objects/organisms may be lost. 11.3. Crystals are usually not found in freshly voided urine but appear after the urine stands for awhile. 11.4. Many of the crystals that are found in the urine have little clinical significance except in cases of metabolic disorders, calculus formation and the regulation of medication. 11.5. Casts are always renal in origin, and they are important indicators of intrinsic renal disease. 11.6. Casts are reported according to type and the number that is present per low power field (10x) 11.7. The accuracy of microscopic identification must be checked by correlation with the macroscopic (i.e., dipstick results), such as the presence of: 11.7.1. Turbidity with Bacteria 11.7.2. Protein with casts, 11.7.3. Positive blood with red cells (RBC) 11.7.4. Positive leukocyte esterase with white cells (WBC) Note: to ensures that all personnel report microscopic morphologic data on patient samples in a similar fashion. For initial accuracy, as well as consistency in serial samples from the same patient, all staff should be consistent with respect to morphologic classification reporting, to accomplish this we will utilize the numeric reporting 05 Jun 05 Rev 2.0 Page 4 of 5 CORELAB, MU-JHU RESEARCH COLLABORATION URINALYSIS – MICROSCOPIC EXAMINATION system of 1+, to 4+ (described above# 9.12) and we will use the CAP urine sediment photomicrographs to ensure all staff review and able to identify Urine microscopy. 12. References 12.1. A Handbook of Routine Urinalysis, Sister Laurine Graft, JB Lippincott Company, ISBN 0-397-52111-1. 12.2. Clinical Diagnosis and Management by Laboratory Methods, 19th Edition, John Bernard Henry, MD, WB Saunders, ISBN 0-7216-6030-4. 12.3. CAP Reference Notebook: Urine, Body Fluids and Misc. 12.4. Urinary Sediment, A Textbook Atlas, Meryl H. Haber, ASCP Press, ISBN 089189-103-X. 13. Appendix 13.1. Urinalysis Worksheet 05 Jun 05 Rev 2.0 Page 5 of 5