Insect sampling in forests and marshes: methods and data
advertisement

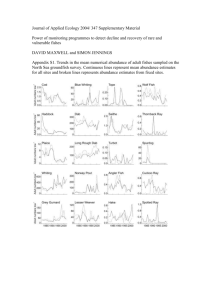
Electronic Supplementary information 1: Insect sampling in forests and marshes: methods and data With: Avian population consequences of climate change are most severe for longdistance migrants in seasonal habitats. Christiaan Both, Chris A.M. van Turnhout, Rob G. Bijlsma, Henk Siepel, Arco J. van Strien, Ruud P.B. Foppen Insect sampling The aim of this analysis is to show whether insect availability shows different temporal patterns during the spring-summer period in forests compared to marshland. There is already some evidence for this in literature, but we gathered additional data to confirm this pattern. This strengthens the power of our comparison between a habitat which we consider highly seasonal (forest) and less seasonal (marshland) in temporal insect abundance. We do not make any statements on whether the seasonality may have changed differently in the different habitats, but we can give a general notion of seasonality, because in both habitats data were collected in several replicates. Data collection Insect biomass was sampled in reed beds in 1992 in two peat-lands, and in 2003 in nine forests across The Netherlands. Although methods of insect sampling necessarily differed between peat-lands and forests, we aim to compare seasonality of insect abundance in these two habitats, rather than comparing absolute abundances. The lowland peat-lands were investigated regarding species composition and abundance of invertebrates. Several vegetation types were incorporated in this research, but here we only present data from reed beds. In total 50 sites, 18 of those being reed beds, have been sampled using emergence traps (as described in Siepel et al. 1989, but we used larger ones here: surface 1 m2 , height 1.4 m, in higher vegetations with an extra base element of 1 m). Emergence traps give a good estimate on the abundance of species. Four types of reed beds (young, dominated by herbs, dominated by Sphagnum, and not inundated) were sampled at two sites in the northern Netherlands and two sites in the central part. Inundated reed beds were only sampled in the northern Netherlands (twice). From May to September traps were operated during four periods of two consecutive weeks each, and samples were collected on a weekly basis. The first two periods covered the main breeding season of marshland birds. Insects were identified to species level if possible. Biomass was measured either directly by dry weight in abundant species, or by interference using length-dry weight ratios in less abundant species (Rogers et al. 1976; Rogers et al. 1977). We then calculated dry weight per m² and vegetation type. As specialist marshland birds mainly forage in reed beds, we focussed on this vegetation type, but the seasonal pattern of relative insect abundance is very similar for other sampled marshlands (like several types of wet grasslands and carrs). We averaged biomass for each sampling period over all areas. Means are presented with their SD. In 2003 we sampled forest insects in nine areas by using three methods (see Both et al. (2006) for selection of the areas). In each area we sampled caterpillar abundance with frass collectors under two Pendiculate oaks Quercus robur. Frass was collected approximately once every five days during the breeding season of pied 1 flycatchers, from late April until mid-June. In some areas with the highest caterpillar biomass we started sampling at, or just after the caterpillar peak (Both et al. 2006), hence the lack of biomass build-up in figure 1c. We also sampled ground-living insects with five pitfall traps in each of the nine areas, located along a transect with two meters between traps. Pitfall traps were emptied once every five days, and insects were stored in alcohol and later sorted by family and size (<2mm, 2-5 mm, 5-10mm, >10mm). Biomass of all insects was calculated on the basis of length-weight relationships, and values were calculated as mg/day for all five traps. Data from the nine areas were averaged as they produced similar patterns with insect abundance peaking at the end of May and declining afterwards. The third method of sampling was restricted to five areas and involved the use of single malaise traps in the main habitat of pied flycatchers. Traps were emptied once every five days, and sorting procedures were similar as for pitfall traps, producing values of insect biomass per trap per 24 hr. Data from the five areas were averaged, but they all give quantitatively similar patterns, showing a rise to a peak in insect abundance at the end of May and a decline afterwards. No samples were collected after mid-June, when all sampling techniques showed declining insect availability. The values for the three different methods cannot be compared quantitatively. Data: Seasonality of insect availability in two habitats In this section we show that forests and marshes differ in the seasonality of insect abundance based on data from The Netherlands, backed-up with published data from other study sites. Forests are known to have a short burst of mainly herbivorous insects that forage on young leaves of deciduous trees before the production of secondary plant compounds starts (Feeny 1970; Southwood et al. 2004). Our measurements in nine forests in The Netherlands corroborated this pattern: insect availability peaked during a couple of weeks in May and June, and declined thereafter (figure 1a-c) (Buse et al. 1999). Caterpillars were most numerous among the insects, but caterpillar peaks differed between sampling areas, and areas with the earliest peak also had the highest peak (Both et al. 2006). The peak of ground-living and flying insects occurred two to three weeks later than the caterpillar peak (figure 1a-b). We chose marshlands as the less seasonal habitat. Marshland is known to have a more extended period of food abundance, reflected in the long breeding season of marsh-inhabiting passerines (see e.g. Halupka et al. 2008). Indeed, our samples from 50 sites in Dutch marshes showed a gradual increase in insect abundance from May until early August (figure 1d). The reason for this longer period of insect abundance is probably because reed (Phragmites australis) continues growing during spring and summer (Dykyjova et al. 1970), and the biomass of herbivorous insects is consequently less peaked (Halupka et al. 2008). Additionally, insects emerge from the water over an extended period in spring and summer (Ward 2005). In conclusion, forests have a stronger seasonality in insect availability than marshes (Ostendorf 1993; Schaefer et al. 2006). Reference List Both, C., Bouwhuis, S., Lessells, C. M. & Visser, M. E. 2006. Climate change and population declines in a long distance migratory bird. Nature 441, 81-83. Buse, A., Dury, S. J., Woodburn, R. J. W., Perrins, C. M. & Good, J. E. G. 1999. Effects of elevated temperature on multi-species interactions: the case of Pedunculate Oak, Winter Moth and Tits. Funct Ecol 13 (Suppl 1), 74-82. 2 Dykyjova, D., Ondok, J. P. & Priban, K. 1970. Seasonal changes in productivity and vertical structure of reed-stands (Phragmites-Communis Trin). Photosynthetica 4, 280-287. Feeny, P. 1970. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51, 565-&. Halupka, L., Dyrcz, A. & Borowiec, M. 2008. Climate change affects breeding of reed warblers Acrocephalus scirpaceus. J. Avian. Biol. 39, 95-100. Ostendorf, W. 1993. Schilf als lebensraum. Beih. Veroff. Naturschutz Landschaftspflege Bad. -Wurtt. 68, 173-280. Rogers, L. E., Buschbom, R. L. & Watson, C. R. 1977. Length-weight relationships of shrub-steppe invertebrates. Ann Ent Soc America 70, 51-53. Rogers, L. E., Hinds, W. T. & Buschbom, R. L. 1976. A general weight vs. length relationship for insects. Ann Ent Soc America 69, 389Schaefer, T., Ledebur, G., Beier, J. & Leisler, B. 2006. Reproductive responses of two related coexisting songbird species to environmental changes: global warming, competition, and population sizes. Journal of Ornithology 147, 47-56. Siepel, H., Meijer, J., Mabelis, A. A. & Den Boer, M. H. 1989. A tool to assess the influence of management practices on the surface macrofauna of grasslands. J. Appl. Ent. 108, 271-290. Southwood, T. R. E., Wint, G. R. W., Kennedy, C. E. J. & Greenwood, S. R. 2004. Seasonality, abundance, species richness and specificity of the phytophagous guild of insects on oak (Quercus) canopies. European Journal of Entomology 101, 43-50. Ward, M. P. 2005. Habitat selection by dispersing yellow-headed blackbirds: evidence of prospecting and the use of public information. Oecologia 145, 650-657. 3 Figure a1: Insect biomass in Dutch forests (a-c) and marshlands (d) during spring and summer. In forests three methods of insect sampling were used: (a) pitfall traps for capturing ground-living insects, (b) malaise traps for capturing flying insects, and (c) frass collectors to measure caterpillar abundance. In the marshes emergence traps were used (d), which sample insects that are present in the vegetation at the moment of placing the trap, as well as insects emerging from the soil or water covered by the trap. The different methods corrupt quantitative comparisons within and across habitats, and we mainly aim to show seasonal variations in insect biomass between habitats. Forests were sampled from late April to mid-June, and marshes between mid-May to mid-September. 4