7.4 Acids & Bases

7.4 Acids and Alkalis
Acids and alkalis are very common substances that are found in the home, in industry and in our bodies. Chemists need to know how to recognise the differences between acids and alkalis and be aware of the chemical reactions they take part in.
7.4.1 Types of solution
All solutions in water are either acidic, alkaline or neutral. Many of the substances you use at home are either acidic, alkaline or neutral.
Strong acids and alkalis of high concentration are corrosive liquids that have to be handled carefully. Acids and alkalis of low concentration are irritants, but care still needs to be taken when using them in the laboratory. Some examples of common acids, alkalis and neutral solutions are shown in the pictures below.
Common acids
Common alkalis
Neutral solutions
TOPIC 7.4: ACIDS AND ALKALIS 1
7.4.2 The pH Scale and Indicators
The pH scale measures how acidic or alkaline a solution is. The scale runs from below 0 to
14 and above. The key things to remember about the scale are:
Acidic solutions have a pH less than 7
Neutral solutions have a pH of 7
Alkaline solutions have a pH greater than 7.
Sometimes you will see the word ‘base’ used instead of alkali, and vice versa. But while they are related to each other, they are not exactly the same thing.
A base is any substance that will neutralise (i.e. cancel out) an acid. An alkali does the same thing, except that an alkali is any base that dissolves in water.
We can tell acidic, alkaline and neutral solutions apart by using special indicators. The indicators change colour depending on whether the solution is acidic, neutral or alkaline.
There are many different types of indicator, but the most common used in the laboratory are litmus and universal indicator.
Litmus
Litmus turns red in acidic solutions and blue in alkaline solutions.
Universal indicator
Universal indicator is a mixture of indicators that has a range of colours depending on the pH of the solution being tested. This makes it a very useful indicator because chemists can work out the precise pH of that solution. Universal indicator is found in most chemistry laboratories and can be used as a solution or as strips of paper.
The figure below shows the pH scale and the colours of universal indicator at different pH values.
Dark red Light red/pink Orange/yellow Green Turquoise/light blue Dark blue Purple
Orange
3 4
Yellow Green
5 6 7 8
Light blue
9
Blue
10 11
Purple
12 13 14
Strong acids Weak acids Neutral Weak alkalis Strong alkalis
TOPIC 7.4: ACIDS AND ALKALIS 2
As the diagram shows, for acids, the lower the pH number, the more acidic the solution, whereas for alkalis the higher the pH number, the more alkaline the solution is.
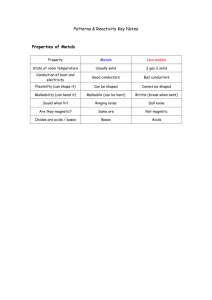
A few other indicators can also be used, and the colours that each one turns at different pH values is summarised in the table below.
Indicator solution
Universal indicator
Litmus paper pH Scale
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Red Orange Yellow Green Light blue Blue Purple
Red Purple Blue
Methyl orange Red Orange Yellow
Phenolphthalein Colourless Pink Red
7.4.3 Acids and alkalis in the laboratory
Many different acids and alkalis are used in the laboratory. Here are some examples of common laboratory acids and alkalis:
Acids
Hydrochloric acid, HCl
Alkalis
Sodium hydroxide, NaOH
Nitric acid, HNO
3
Sulphuric acid, H
2
SO
4
Limewater, Ca(OH)
2
Ammonium hydroxide, NH
4
OH
All of the acids and alkalis used in the laboratory can be dangerous, so all bottles of these substances will show the CORROSIVE hazard warning sign. Always be careful when handling any solutions in the lab. Wear eye protection at all times, wash any spills with lots of water and report all incidents to your teacher.
TOPIC 7.4: ACIDS AND ALKALIS 3
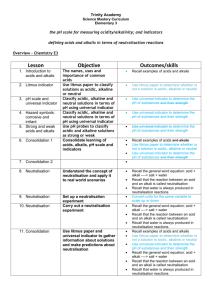
7.4.4 Neutralisation
When an acid is added to an alkali, or vice versa, they cancel each other out. If the correct amounts of each are added, a neutral solution is formed. This kind of chemical reaction is called neutralisation. It is a common reaction in the lab and happens in many situations in real life. We can follow a neutralisation reaction by using an indicator. In the example below, sodium hydroxide is being added to hydrochloric acid. Universal indicator is used to follow the reaction.
Sodium hydroxide (pH 14)
Universal indicator is present in both of these solutions.
Sodium chloride (neutral, pH7)
Hydrochloric acid (pH 1)
As the alkali is added the solution becomes less acidic and the pH increases towards 7
(neutral). We have to be careful to add the exact amount of alkali needed to neutralise the acid. If we add too much alkali the solution will become alkaline. If we add too little alkali the solution will still be acidic.
The same reaction happens if it’s done the other way around. As the acid is added the solution becomes less alkaline and the pH falls towards 7 (neutral).
7.4.5 Neutralisation in the laboratory
The volume of acid and alkali required for neutralisation depends on the nature and concentration of each solution. To follow a neutralisation reaction, chemists use special apparatus to measure the volumes of liquids accurately. The technique is called a
titration.
TOPIC 7.4: ACIDS AND ALKALIS 4
7.4.6 Neutralisation in real life
Some examples of neutralisation in real life are listed below:
1. Some plants don’t grow well in acidic soils, so farmers and gardeners add an alkali to lower the acidity (raise the pH) of the soil. The alkali they add is called lime, and by reducing the acidity of the soil the plants grow better.
2. Wasp stings are alkaline, so we treat them with a weak acid such as vinegar, which neutralises the sting.
3. Bee stings are acidic, so we need to add a weak to neutralise it, such as baking soda (sodium bicarbonate). alkaline substance
4. Too much acid in the stomach causes indigestion. To treat it we take indigestion tablets to neutralise the excess acid. The tablets can contain alkalis such as magnesium hydroxide, Mg(OH)
2
, and calcium carbonate, CaCO
3
.
7.4.7 Reactions of acids and bases
A base is a substance that reacts with an acid to produce a salt and water. This is a
neutralisation reaction. An alkali is a base that can dissolve in water. Bases are the opposite of acids and are sometimes called antacids or neutralisers. Metals (e.g. iron, magnesium), metal oxides (e.g. copper oxide), metal hydroxides (e.g. sodium hydroxide) and metal carbonates (e.g. copper carbonate) are all examples of bases.
Word equations: acid + base salt + water acid + alkali salt + water acid + metal salt + hydrogen acid + metal oxide salt + water acid + metal carbonate salt + water + carbon dioxide
TOPIC 7.4: ACIDS AND ALKALIS 5
The name of a salt is determined by the acid and base used. Hydrochloric acid produces chlorides , sulphuric acid produces sulphates and nitric acid produces nitrates . Examples are: hydrochloric acid + sodium hydroxide sodium chloride + water sulphuric acid + copper oxide copper sulphate + water nitric acid + copper oxide copper nitrate + water
Some metals also react with acids to produce salts. Reactions with metals produce hydrogen gas as another product. This can be identified by holding a lit splint to a tube of the gas. A ‘squeaky pop’ or ‘squeaky explosion’ indicates that hydrogen is present. acid + metal salt + hydrogen
For the reaction of a metal carbonate and acid, one of the products is always carbon dioxide, CO
2
. acid + metal carbonate salt + carbon dioxide + water
Carbon dioxide can be identified by bubbling the gas through limewater. If carbon dioxide is present, the limewater will turn cloudy.
Rainwater is naturally acidic as carbon dioxide gas from the air dissolves into water in the clouds. Some polluting gases such as sulphur dioxide (SO
2
) dissolve in rainwater to make it more acidic. Acid rain is rainwater that has a pH significantly less than 7.
TOPIC 7.4: ACIDS AND ALKALIS 6
TOPIC SUMMARY
Acids and alkalis are types of solution which are found in the laboratory or at home.
Strong acids and alkalis are ____________ and need to be handled with care.
_____________ are substances which change colour in acids and alkalis.
Universal indicator has a range of __________ depending on the pH of the solution. An
________ has a pH of below 7 and an ___________ has a pH of above 7. A
____________ solution has a pH of ________.
A ______________ reaction occurs when an acid and alkali cancel each other out. This requires a carefully measured volume of each, this technique is usually called a
__________________. This process needs a very sharp colour change and the indicators used are usually methyl orange or ___________________.
Neutralisation reactions are important and occur in many everyday situations. Farmers may use a base to neutralise _________ soils. People may use __________ tablets to treat indigestion. ___________ may be used to treat an alkali wasp sting.
When acids and alkalis react together the products are salt and __________. The type of salt produced depends on the name of the alkali and the type of acid used. For example sulphuric acid would make a sulphate, nitric acid would make a ____________ and hydrochloric acid would produce a ____________.
Acids also react with some ________, metal oxides and metal carbonates. A summary of these reactions is:
Metal + acid salt + _____________
Metal oxide + acid _________+ water
Metal carbonate + acid salt + water + ______ ____________ neutral acid acidic indicators corrosive alkali colours antacid 7 titration salt hydrogen chloride nitrate carbon dioxide water vinegar neutralisation metals phenolphthalein
TOPIC 7.4: ACIDS AND ALKALIS 7