115Exam2redo
advertisement

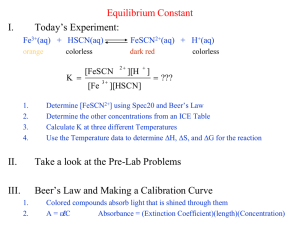
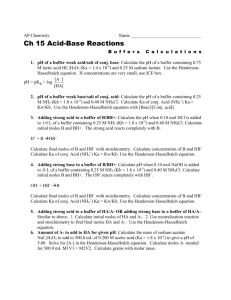
June 20, 2005 Dr. Nash 85 Points Possible Chemistry 115 Exam 2 (redo) NAME:__________________ Potentially useful constant: R= 8.314 J/Mol K= 0.08206 Latm/mol K 1. At 25 C, the pH of a 0.097 M solution of cyanic acid HSCN is 3.88. (a) What is the percent dissociation of this 0.097 M solution? (b) What is the Ka of HSCN (c) What is the percent dissociation of HSCN is a 0.194 M solution? (d) What is the pH of a 0.194 M solution of HSCN (e) What is the Kb of KSCN? (f) What is the pH of a 0.097 M solution of KSCN? 2. A buffer solution is prepared by dissolving .125 moles of HSCN and 0.100 moles of NaSCN in 1.2 L of solution. (a) What is the pH of this buffer solution? (b) What is the pH of the buffer solution after 0.45 moles of HCl(g) is dissolved in this 1.2 L of buffer? (assume the volume of the solution does not change) (c) What would the pH of the buffer solution have been in 1.00 moles of NaOH was dissolved in 1.2 L of the buffer (again, no volume change) (d) The pH of a buffer solution prepared using the same two species (HSCN and SCN- is 5.02. What is the ratio of [HSCN] to [SCN-] in this solution? 3. Consider the data below for the following equilibrium at 25 C Np(OH)3 Np3+ + 3 OHName Np(OH)3 (s) Np3+(aq) OH-(aq) Gof (kJ/mol) -835.0 -245.0 -157.0 (a) What is Go for this process? (b) What is Ksp for this process? (c) What is the solubility of Np(OH)3 in water? (d) What is the solubility of Np(OH)3 in a solution buffered to pH 11.00? 4. Consider the following data for a reaction taking place in an acidic solution phase at 25C. CH3I(aq) + H2O(l) CH3OH(aq) + HI(aq) Experiment 1 2 3 4 [CH3I] 0.100 0.200 0.200 0.400 [H+] 0.01 0.10 0.01 0.10 rate Ms-1 0.0137 0.0274 0.0274 0.0548 (a) What is the rate law and overall order of this reaction? (b) What is the value of the rate constant for this reaction? (c) What would the rate of the reaction be if the concentration of CH3I in acid solution is 0.05 M? 5. Consider the following reaction which takes place in the gas phase CH3Br(g) + H2O(g) CH3OH(g) + HBr(g). with the following data: Note: it is observed that in this case, H2O does not explicitly appear in the rate law: 25C time(s) [CH3I] 0 10 20 30 40 50 60 80 90 100 110 120 130 140 150 160 170 180 190 200 37C [CH3I] 3.0000 1.1321 0.6977 0.5042 0.3947 0.3243 0.2752 0.2113 0.1893 0.1714 0.1567 0.1442 0.1336 0.1245 0.1165 0.1095 0.1033 0.0977 0.0927 0.0882 3.0000 0.9677 0.5769 0.4110 0.3191 0.2609 0.2206 0.1685 0.1508 0.1364 0.1245 0.1145 0.1060 0.0987 0.0923 0.0867 0.0817 0.0773 0.0733 0.0698 (a) What is the order of this reaction? Show your work. (b) What is the rate constant at 25 C? (c) What is the (first) half-life of CH3I at 25 C? (d) How long will it take at 25 C for [CH3I] to reach 0.0010 M (e) What is the rate constant at 37 C (f) What is the activation energy of this reaction? (g) What would the rate constant be for this reaction at 40 C? Bonus: Tritium is a radioactive isotope of hydrogen (3H) used in triggers for thermonuclear weapons. It decays by beta decay (emission of a nuclear electron) with a half-life of 8.25 years. A trigger for a thermonuclear device can function when at least 33% of its original supply of tritium remains in the ‘device’. So, how often does the tritium in a thermonuclear device need to be changed? Potentially Useful Equations: K H o ln 2 R K1 1 1 T2 T1 k Ea 1 1 ln 2 R T2 T1 k1 Ea k Ae RT 1 1 kt At A 0 ln A t ln A0 kt G G RT ln Q o At A0 e kt