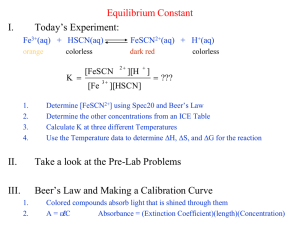
Determination of an Equilibrium Constant 𝐹𝑒 3+ (𝑎𝑞) + 𝐻𝑆𝐶𝑁 (𝑎𝑞) ↔ 𝐹𝑒𝑆𝐶𝑁 2+ (𝑎𝑞) + 𝐻 + (𝑎𝑞) 𝑘𝑒𝑞 = [𝐹𝑒𝑆𝐶𝑁 2+ ][𝐻 + ] [𝐹𝑒 3+ ][𝐻𝑆𝐶𝑁] Victoria Alexis Treto 02/22/2018 Che122, General Chemistry 2 Spring 2018 Prof. L. Wojciechowicz Introduction The purpose of this lab is to evaluate the equilibrium constant of the reaction between ferric ions and hydrogen thiocyanate by determining the concentration of Procedure Preparation of the HSCN Solution Step1: From a repipet bottle, 10.0ml of a 0.01M KSCN solution was added to a clean 50.00ml volumetric flask. This was then diluted to the mark with 0.5M HNO3, mixed well, and transferred to a labeled beaker. Preparation of the Beer’s Law Calibration Plot for the FESCN2+ Solutions Step1: First, 4 clean 25.00ml volumetric flasks were labeled #1-4. Using pipettes, all the standard solutions were prepared as described in the table. 5.00ml of the 0.200M iron nitrate solution was transferred to a 25.00ml volumetric flask and the appropriate HSCN amount was added. This was then diluted to the mark with 0.5M Nitric acid and mixed thoroughly. The absorbance of all solutions was measured at 447nm and 0.5M HNO3 was used for the blank. Determination of FESCN2+ at Equilibrium Step1: Another 4 clean volumetric flasks were labeled #1-4. Using pipettes, the equilibrium solutions as described in the table in our lab manual were prepared. 2.00X10-3M iron nitrate was transferred to a 10.00ml volumetric flask and the appropriate amount of HSCN was added. This was then diluted to the mark with HNO3. Absorbance for all solutions was measures at 447nm. Data/Calculations Part1: Standard Solutions Sol’n# ml HSCN A 1 2 3 4 0.50ml 1.00ml 1.80ml 2.50ml 0.168 0.318 0.597 0.794 Initial M HSCN 0.00200 0.00400 0.00600 0.00800 Molarity FeSCN2+ 0.000040 0.000080 0.000144 0.000200 Part2 A: Equilibrium Solutions Sol’n# 1 2 3 4 Ml HSCN 1.00ml 2.00ml 3.00ml 4.00ml A 0.120 0.217 0.318 0.426 Molarity FeSCN2+ 0.0000324 0.0000569 0.0000823 0.0001100 Equation attained from Calibration Chart: y=3969.7x+0.0088 𝑺𝒐𝒍′ 𝒏#𝟏 𝑴𝒆𝒒 𝑭𝒆𝑺𝑪𝑵𝟐+ = 𝑺𝒐𝒍′ 𝒏#𝟒 𝑴𝒆𝒒 𝑭𝒆𝑺𝑪𝑵𝟐+ 𝟑𝟗𝟔𝟗.𝟕 = 𝟑. 𝟐𝟒 × 𝟏0−5 (𝟎. 𝟐𝟏𝟕 + 𝟎. 𝟎𝟎𝟖𝟖) = 𝟓. 𝟔𝟗 × 𝟏𝟎−𝟓 𝟑𝟗𝟔𝟗. 𝟕 (𝟎. 𝟑𝟏𝟖 + 𝟎. 𝟎𝟎𝟖𝟖) = = 𝟖. 𝟐𝟑 × 𝟏𝟎−𝟓 𝟑𝟗𝟔𝟗. 𝟕 (𝟎. 𝟒𝟐𝟔 + 𝟎. 𝟎𝟎𝟖𝟖) = = 𝟏. 𝟏𝟎 × 𝟏𝟎−𝟒 𝟑𝟗𝟔𝟗. 𝟕 𝑺𝒐𝒍′ 𝒏#𝟐 𝑴𝒆𝒒 𝑭𝒆𝑺𝑪𝑵𝟐+ = 𝑺𝒐𝒍′ 𝒏#𝟑 𝑴𝒆𝒒 𝑭𝒆𝑺𝑪𝑵𝟐+ (𝟎.𝟏𝟐𝟎+𝟎.𝟎𝟎𝟖𝟖) Part2 B: Equilibrium Constant Values F𝒆𝟑+ Sol’n# + HSCN FeSCN𝟐+ ← − − −→ + 𝑯+ Initial M 1.00x1𝟎−𝟑 2.00x1𝟎−𝟒 0.00 0.500 ∆𝐌 -3.24x1𝟎−𝟓 -3.24x1𝟎−𝟓 +3.24x1𝟎−𝟓 0 Equil. M 9.68x1𝟎−𝟒 1.68x1𝟎−𝟒 3.24x1𝟎−𝟓 0.500 1 𝟎. 𝟓𝟎𝟎 × 𝟑. 𝟐𝟒𝒙𝟏𝟎−𝟓 𝑲𝒄 = = 𝟗𝟗. 𝟔 𝟎. 𝟎𝟎𝟎𝟗𝟔𝟖 × 𝟎. 𝟎𝟎𝟎𝟏𝟔𝟖 F𝒆𝟑+ Sol’n# + Initial M 1.00x1𝟎−𝟑 FeSCN𝟐+ ← − − −→ + 𝑯+ 2.00x1𝟎−𝟒 0.00 0.500 -5.69x1𝟎−𝟓 -5.69x1𝟎−𝟓 +5.69x1𝟎−𝟓 0 Equil. M 9.43x1𝟎−𝟒 1.43x1𝟎−𝟒 5.69x1𝟎−𝟓 0.500 ∆𝐌 2 𝑲𝒄 = F𝒆𝟑+ Sol’n# 𝟎. 𝟓𝟎𝟎 × 𝟓. 𝟔𝟗𝒙𝟏𝟎−𝟓 = 𝟐𝟏𝟏. 𝟎 𝟎. 𝟎𝟎𝟎𝟗𝟒𝟑 × 𝟎. 𝟎𝟎𝟎𝟏𝟒𝟑 + Initial M 1.00x1𝟎−𝟑 HSCN FeSCN𝟐+ ← − − −→ + 𝑯+ 2.00x1𝟎−𝟒 0.00 0.500 -8.23x1𝟎−𝟓 -8.23x1𝟎−𝟓 +8.23x1𝟎−𝟓 0 Equil. M 9.18x1𝟎−𝟒 1.18x1𝟎−𝟒 8.23x1𝟎−𝟓 0.500 ∆𝐌 3 𝑲𝒄 = F𝒆𝟑+ Sol’n# 𝟎. 𝟓𝟎𝟎 × 𝟖. 𝟐𝟑𝒙𝟏𝟎−𝟓 = 𝟑𝟖𝟐. 𝟎 𝟎. 𝟎𝟎𝟎𝟗𝟏𝟑 × 𝟎. 𝟎𝟎𝟎𝟏𝟏𝟖 + Initial M 1.00x1𝟎−𝟑 4 HSCN HSCN FeSCN𝟐+ ← − − −→ + 𝑯+ 2.00x1𝟎−𝟒 0.00 0.500 -1.10x1𝟎−𝟒 -1.10x1𝟎−𝟒 +1.10x1𝟎−𝟒 0 Equil. M 8.90x1𝟎−𝟒 9.00x1𝟎−𝟓 1.10x1𝟎−𝟒 0.500 ∆𝐌 𝟎. 𝟓𝟎𝟎 × 𝟏. 𝟏𝟎𝒙𝟏𝟎−𝟒 𝑲𝒄 = = 𝟔𝟖𝟔. 𝟔 𝟎. 𝟎𝟎𝟎𝟖𝟗𝟎 × 𝟎. 𝟎𝟎𝟎𝟎𝟗 Mean Kc= 𝟗𝟗.𝟔+𝟐𝟐𝟏.𝟎+𝟑𝟖𝟐.𝟎+𝟔𝟖𝟔.𝟔 𝟒 = 347.3 RAD 347.3-99.6=247.7 347.3-211.0=136.3 347.3-382.0=34.7 327.3-686.6=359.3 Avg. Deviation 𝟐𝟒𝟕. 𝟕 + 𝟏𝟑𝟔. 𝟔 + 𝟑𝟒. 𝟕 + 𝟑𝟓𝟗. 𝟑 = 𝟏𝟗𝟒. 𝟔 𝟒 RAD RAD= 𝟏𝟗𝟒.𝟔 𝟑𝟒𝟕.𝟑 = 𝟎. 𝟓𝟔 = 𝟓𝟔% Discussion When plotting the absorbance versus moles of FeSCN2+, the equation came out to be y=3969.7x+0.0088 which seems accurate when comparing it to similar experiments’ equations. From that equation, attaining the equilibrium moles for FeSCN2+ was intuitive. There is lots of room for error in terms of precision for this lab. Look at my rate of deviation. When I was getting varying Kc values, I could tell something was amiss. When transferring the HSCN, the amounts could’ve been mis-measured and that could account for a different absorbances than needed, and thus affecting the equilibrium constants and kc values simultaneously. Overall, this lab could’ve been executed in a more accurate way myself, but now have a more optimal understanding of how to use ICE tables to calculate the concentration at equilibrium for a specified solution.