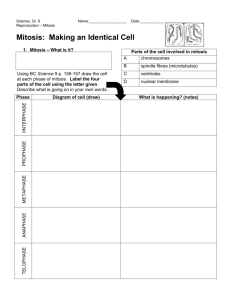
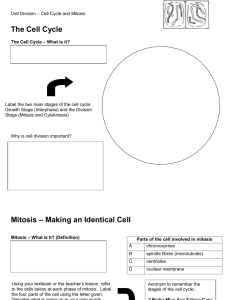
During the cell cycle, cells progress through four distinct phases: M
advertisement

Sarah Cole News & Views February 24, 2004 Cell Cycle: Plk1 Destruction Leaves a Cell Divided Progression through the cell cycle is highly regulated, and abnormalities in cell cycle progression are a frequent cause of tumorigenesis. Understanding the cell cycle and its regulation may therefore provide understanding about how normal cells become cancerous and how to develop new therapies (reviewed in Park and Lee, 2003). During the cell cycle, cells progress through four distinct phases: M, G1, S and G2. Mitosis, or M phase, is divided into five sub-phases: prophase, prometaphase, metaphase, anaphase and telophase. Transitions from one phase to another are monitored by checkpoints, signaling pathways that respond to internal and external cues to prevent cell cycle progression until conditions are favorable. The transition out of M phase into G1 is known as the exit from mitosis and is monitored by the metaphase checkpoint, which delays the separation of replicated chromatin until all the chromosomes are aligned on the mitotic spindle. The metaphase checkpoint is controlled by the degradation of cyclins, proteins named for their cyclic expression during the cell cycle. Protein degradation is carried out by a protease, the 26S proteasome. The proteasome targets proteins that have been tagged with a chain of short peptides called ubiquitin. During mitosis, cyclin polyubiquitination depends on activation of the anaphase promoting complex (APC), a complex of proteins activated through its binding to another protein, Cdc20, during prometaphase. The kinase Cdk1 enhances Cdc20 binding to the APC by phosphorylating several of the APC subunits, and at the same time Cdk1 phosphorylation of another protein, Cdh1, prevents it from interacting with the APC (reviewed in Peters, 2002). In budding yeast, after Cdc20 activation of the APC begins cyclin ubiquitination and degradation, phosphotase Cdc14 is activated, and it dephosphorylates Cdh1 and inactivates Cdc28, the budding yeast Cdk1 homologue (Visintin et al., 1998). Cdh1 is now able to bind the APC. The switch from an APC bound to Cdc20 to an APC bound to Cdh1 is thought to allow other substrates to be degraded. Cdc14 and Cdh1 activation is regulated by the mitotic exit network (MEN) signal transduction pathway (reviewed in Bardin and Amon, 2001). An MEN in mammals, however, has not yet been identified and the regulation of protein degradation by Cdh1 bound APC and mitotic exit is less understood. Lindon and Pines show that APC-Cdh1 mitotic substrates are degraded at different rates and at different times during exit from mitosis. Using fluorescent tagged proteins and examining their activities in living mammalian cells, they show that proteolysis of one of these substrates, Plk1, begins in anaphase but only after the APC has switched from binding Cdc20 to binding Cdh1. Furthermore, Plk1 degradation occurs via the proteasome. Plk1 is a kinase known to play a role in assembly of the contractile ring during cytokinesis and in the metaphase to anaphase transition, and it is activated during mitosis by phosphorylation (Carmena et al., 1998; Seong et al., 2002; Kelm et al., 2002). But is the APC-Cdh1 mediated proteolysis of Plk1 needed for mitotic exit? To answer this question, the authors expressed a nondegradable form of Plk1 in mammalian cells and showed that both mitotic exit and cytokinesis are delayed. Similarly, cells expressing a constitutively active form of Plk1 that cannot be dephosphorylated also showed delayed mitotic exit. These results imply that Plk1 is inactivated during anaphase by degradation or dephosphorylation in order for normal exit from mitosis to occur. Given Plk1’s role in cytokinesis, these experiments suggest that the inactivation of Plk1 during anaphase assists in preventing premature cytokinesis. Lindon and Pines therefore provide a link between proteolysis during mitotic exit and the regulation of cytokinesis in mammalian cells. Their results show that a signaling pathway may regulate mitotic exit and APC-Cdh1 activation, as the MEN does in budding yeast. References Bardin, A.J. and Amon, A. 2001. Men and sin: what's the difference?. Nat. Rev. Mol. Cell. Biol. 2:815-826. Carmena, M., M.G. Riparbelli, G. Minestrini, A.M. Tavares, R. Adams, G. Callaini, and D.M. Glover. 1998. Drosophila polo kinase is required for cytokinesis. J. Cell Biol. 143:659–671. Kelm, O., M. Wind, W.D. Lehmann, and E.A. Nigg. 2002. Cell cycle-regulated phosphorylation of the Xenopus polo-like kinase Plx1. J. Biol. Chem. 277:25247–25256. Park, M.T. and Lee S.J. 2003. Cell cycle and cancer. J. Biochem. Mol. Biol. 36(1):60-5. Peters, J.M. 2002. The Anaphase-Promoting Complex: Proteolysis in Mitosis and Beyond. Mol. Cell 9:931-943. Seong, Y.S., K. Kamijo, J.S. Lee, E. Fernandez, R. Kuriyama, T. Miki, and K.S. Lee. 2002. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J. Biol. Chem. 277:32282–32293 Visintin, R., Craig, K., Hwang, E.S., Prinz, S., Tyers, M., and Amon, A. 1998. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2:709-718.