Arterial Blood Gas Measurements
advertisement

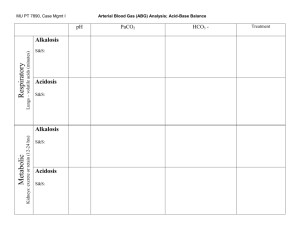
How To Quickly Analyze Arterial Blood Gas Measurements (ABGs) Use ABGs to evaluate the acid-base balance in the body. Balance is crucial to normal functioning of the body's systems; severe imbalances can be lethal. Here's an easy 4-step method of evaluation. Difficulty: Hard Time Required: 5 minutes Here's How: 1. Key concept #1: pH is an expression of the free hydrogen ion concentration of a substance. Blood has a normal pH of 7.35 to 7.45. 2. Key concept #2: An increase in hydrogen ion concentration lowers pH and increases acidity. A decrease in hydrogen ion concentration raises pH and decreases acidity. 3. Key concept #3: The body doesn't fully compensate for a primary acid-base disorder; in compensated acidosis the pH will be below 7.4, and in compensated alkalosis it will be above 7.4. 4. Key concept #4: The PaCO2, or partial pressure of carbon dioxide, is the respiratory component of acid-base balance. Normal values are 35 to 45 mmHg. 5. Key concept #5: The HCO3, or bicarbonate, is the metabolic component of acid-base balance. Normal values are 22 to 28 mEq/L. 6. Step 1. Evaluate the blood pH. Is the pH within the normal range of 7.35-7.45? If so, the patient is either normal or compensated. Does the pH tend to the acid or alkaline side? 7. Is the patient acidotic, with a pH of less than 7.35, or alkalotic, with a pH of greater than 7.45? 8. Step 2. Evaluate the PaCO2. Is the PaCO2 normal, between 35 and 45 mmHg? Is it below 35, indicating an alkaline condition? Is it above 45, indicating an acidic condition? 9. Step 3. Evaluate the HCO3. Is the HCO3 normal, between 22 to 28 mEq/L? Is it above 28, indicating an alkaline condition? Is it below 22, indicating an acidic condition? 10. Step 4. Put it together. Ask which component, the CO2 or HCO3, matches the pH acid-base state. Decide if the patient has respiratory acidosis or alkalosis, or metabolic acidosis or alkalosis. 11. Example: pH 7.31 (acidic), CO2 60 (acidic) and HCO3 23 (normal). The patient has respiratory acidosis, since the CO2's acidic state matches the pH's acidic state and CO2 is the respiratory component. 12. Example: pH 7.58 (alkaline) CO2 37 (normal), HCO3 31 (alkaline). Condition is metabolic alkalosis, as the HCO3 matches the pH's alkaline state and HCO3 is the metabolic component. 13. Example: pH 7.44 (alkaline side of normal) CO2 29 (alkaline) HCO3 20 (acid). Condition is respiratory alkalosis, as the CO2's alkaline state matches the pH. 14. Example: pH 7.35 (acidic side of normal) CO2 30 (alkaline) and HCO3 18 (acidic). Condition is metabolic acidosis. 15. Example: pH 7.39 (acidic side of normal), CO2 39 (normal), HCO3 25 (normal). Normal ABG's. (Once in a while it happens!) Tips: 1. If an abnormal pH exists, the body will try to correct it. The respiratory system starts CO2 correction quickly, within minutes. HCO3 correction via the kidneys is much slower, taking hours to begin. 2. If the pH is abnormal, one value (CO2 or HCO3) is abnormal and one value is normal, the condition is uncompensated – the other value has not yet started to neutralize the abnormal pH. 3. If the pH is abnormal and both the CO2 and HCO3 are abnormal, the condition is partially compensated. The body has started neutralizing the abnormal pH but compensation is not complete. 4. If the pH is normal and both the CO2 and HCO3 are abnormal, the condition is compensated. The body has compensated for the abnormal pH. 5. If the pH, CO2 and HCO3 are normal and were previously abnormal, the ABG is called corrected.