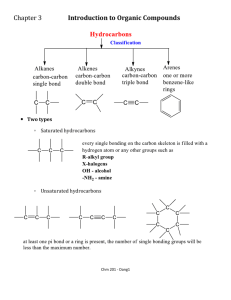
SECTION B
advertisement

SECTION B PROGRAMMES/SUB-DISCIPLINE/ DISCIPLINE TO BE ACCREDITED 36. Title of programme/sub-discipline/discipline to be accredited: BACHELOR OF TECHNOLOGY DEGREE IN PURE AND APPLIED CHEMISTRY TYPE OF ACCREDITATION REQUIRED 37. Indicate below the type of accreditation required Please tick ( ) one Initial Accreditation 37.2 38. Re-accreditation Has any NUC Accreditation Panel visited your University to determine if the programme/sub-discipline/discipline can be accredited. Please tick ( ) one Yes No 39. If answer to item 38 is YES, please attach to the completed Form a photocopy of main decision and recommendations of the Commission? 40. Name of Faculty/School/College in which the programme/sub-discipline/discipline to be accredited is offered: FACULTY OF PURE AND APPLIED SCIENCES 40.1 Name of Department: DEPARTMENT OF PURE AND APPLIED CHEMISTRY 41. Date of establishment of Department: APRIL 1990 42. Name and Qualification (s) of Dean of Faculty/or Provost/Dean of College/School: PROFESSOR E. T. AYODELE, B.Sc., M.Sc., Ph.D. 43. Name and qualification(s) of Head of Department offering the programme to be accredited. DR. I.O. ADEOYE, B.Sc., M.Sc., Ph.D. 1 44. HISTORY OF THE PROGRAMME/SUB-DISCIPLINE/DISCIPLINE Write below, a brief history of the development of the Programme/Sub-discipline/Discipline to be accredited. Academic Programmes The department of Pure and Applied Chemistry is one of the foundation departments of the Faculty of Pure and Applied Sciences of Ladoke Akintola University of Technology Ogbomoso, Oyo State, Nigeria, when it began in 1990. The Department teaches students for the Degree of Bachelor of Technology in Pure and Applied Chemistry. The programme has its developments based on providing the basic required exposure on both teaching and research towards day-to-day growth in the field of Science and Technology viz-a-viz Physical, Organic, Inorganic Chemistry, Industrial and Analytical Chemistry. It produced its first batch of graduates in 1997 and in the 1997/1998 session its postgraduate programmes began. Ever since, the department has continued to grow in quality and quantity. The student population grew from the humble beginning of about 30 students at inception to 450 now and has continued to grow. Equally, the staff strength grew from six to twenty-seven. It is also pertinent to note that at the inception, there was only a single professor in the department, while today six professors have emerged, with five of them being founding members. Worthy of note also is that the department has two well equipped laboratories with the third at about 90% completion on–going. It is expected that at completion, it is going to be one of the best in the University. ADMINISTRATION IN GENERAL OF PROGRAMME/ SUB-DISCIPLINE/DISCIPLINE 45. Describe how the programme/sub-discipline/discipline is administered. Use separate sheets. The description should highlight the following: 45.1 Personnel Administration (a) Organization Structure: While the Head of Department is responsible for the overall running of the Department, the academic staff that have been allocated specific responsibilities such as Examination officer, Registration officer/ Level adviser, Curriculum officer and so on assist him/her. Various committees are set up to assist the Head. The Departmental Secretary oversees the running of the Departmental Office and supervises the rest of the administrative staff. The Technical staff (who operate directly under the Chief Technologist) assist the Laboratory Supervisors (Academic Staff) in the running of the Laboratory practical, but is responsible to the Head of Department in matters concerning procurement major repairs and maintenance and staff administration. (See the Chart Below) (b) Head of Department Academic Technical Administrative Lecturers of all cadres Chief Technologists Secretary Technologists Laoboratory Supervisors/ Assistants 2 Data Management Officer Office Assistant Typist (c) How staff are involved in the decision-making process and in general administration: Staff members are involved in the decision making process through their participation in departmental and various committee meetings. As shown in the organizational structure, each staff is assigned some responsibilities directly or through membership of a committee within the department or in representing the department in some Faculty/University committee. In this way, the staff are involved in the general administration of the department, the Faculty and the University at large. (d) Policy and Practice of Staff Development: The Department, in line with University policy on staff development strives always to adopt a policy that provides conducive atmosphere for staff in the discharge of their duties and is also involved in the following: (i) recruitment and training of graduate assistants (ii) in-service training of staff/ postdoctoral fellowship (iii) teaching and research (iv) granting of training and sabbatical leave. (e) Staff Promotion The process of appraisal of staff for promotion is as follows: 45.2 (i) The staff is appraised by his/her immediate supervisor or Head of Department. The staff reads and either endorses his acceptance or otherwise of the appraisal. (ii) The Departmental Review Committee discusses the individual staff appraisals and forwards same with its recommendations to the Faculty Review Panel Committee. (iii) The Faculty Review Panel considers the Departmental recommendations and forwards same with its own recommendations to the University Appointments and Promotions Committee. (iv) The University Appointments and Promotions Committee gives the final approval to recommendations from Faculty Review Panel, Administrative/ Technical Staff Review Committee in respect of all promotions, except those to the ranks of Associate Professor (Reader) and Professor, which are first sent out for external assessment before approval. Students’ Welfare (a) Handling of Academic Grievances: Grievances of students which may involve the generality or a group of students can be raised either through the representative of a class or by the leadership of the Students’ Chemical Society of Nigeria (SCSN), LAUTECH Chapter. Complaints are first made to the level coordinators or the student advisers, who may be able to dispose of the matter or direct the students to the Head of Department. An aggrieved individual student could also follow the same channel for the resolution of his or her case. Students could also use either the Departmental or Faculty student-staff forum to discuss their grievances. 3 (b) Students Academic Advising: Students could get advice on academic matters from any of the following: the course lecturer, the class registration officer, the student adviser, level coordinator or the Head of Department. The Counseling unit (Students Affairs Office) is also available for general counseling, including academic. 45.3 Examination (a) Setting The lecturers who taught the course set the examination questions. The questions are read and moderated by a senior colleague within the department. (b) Conduct Examinations are administered at the end of the semester during which the course is taught. The examinations are conducted in accordance with the senateapproved university examinations regulations. (c) Evaluation Schemes A student’s performance in a course is evaluated based on marks scored in the final examination and in his continuous assessment. The continuous assessment is given a weighting of 30% while the final examination has a weighting of 70%. The minimum pass mark is 40% and the student who fails the course is allowed to register for the course again and take the examination when next it is offered, in accordance with the Grade Point System. (d) Moderation Schemes 100 level – 400 level examinations are moderated internally as described in (a) above, while 500 level degree examinations are moderated by an External Examiner from another university approved by Senate. The External Examiner moderates both the question papers and marking schemes before the examination is taken and reviews the students’ performance and grading after the examination has been marked. (e) Issuance of Results Results are first considered at the meeting of the departmental board of examiners before presentation to the faculty board of examiners for recommendation to Senate for Approval. After Senate approval, the Academic Office of the University releases the results. Letter grades and Grade points are used in the presentation of the results as follows: % Score Letter Grade Grade Point Remark 70-100 A 5 Pass 60-69 B 4 Pass 50-59 C 3 Pass 45-49 D 2 Pass 40-44 E 1 Pass -------------------------------------------------------------------------------0 – 39 F 0 Fail At the end of the session the Cumulative Grade Point Average CGPA, is computed as per the National University Commission Minimum Academic Standards Curriculum and released to the student: the class of degree of a graduating student is determined as follows: 4 Class of Degree 1st Class 2nd Class Upper 2nd Class Lower 3rd Class Pass 45.4 CGPA 4.50 - 5.00 3.50 - 4.49 2.40 - 3.49 1.50 - 2.39 1.00 - 1.49 Academic Atmosphere (a) Any policy adopted and practiced by the College/School/Faculty/Department in pursuit of academic Standards and maintenance of academic atmosphere Students who excel at the end of the academic session are recognized and awarded prizes during the convocation. Staff and students meet at seminars, presentation of project and SIWES reports. DEGREE OFFERED: Bachelor of Technology, B.Tech (Pure and Applied Chemistry) REQUIREMENTS FOR THE AWARD OF THE DEGREE To be eligible for the award of the B.Tech (Pure and Applied Chemistry) degree, a candidate must spend the minimum number of semesters prescribed by the University and must in addition, pass all University/ Faculty compulsory courses and all departmental compulsory courses as well as the option/ restricted electives and free electives as prescribed for the degree. EVALUATION OF STUDENTS’ PERFORMANCE EXAMINATIONS: Examinations are conducted in accordance with regulations approved from time to time by the University Senate. To sit for any end-of-course examination, candidates must be duly registered for the course and attain 75% point in attendance at the course lectures/ laboratory practical/ tutorials. Students who are absent from lectures/ laboratories/ tutorials must communicate their reasons to their course lecturers. Every course shall be examined during the academic semester for which it is taken. End of course examination will consist of one or more of the following: (a) written examination – 70% (b) practical and/or continuous assessments – 30% Note that continuous assessment is for all courses taught. GRADING SYSTEM The following letters grades are attached for the examination scores: % MARK 70 – 100 60 – 59 50 – 59 45 – 49 40 – 44 39 – 0 LETTER GRADE A B C D E F GRADE POINT 5 4 3 2 1 0 The minimum pass mark for each course is 40% that is grade “E”. 5 CREDIT LOADS REQUIREMENTS The maximum credit hour for any one session is 48 and the minimum is 36 or as specified in the curriculum. AWARD OF DEGREE For the award of the B.Tech. Degree in Chemistry, a student must successfully complete the prescribed courses and obtain a cumulative grade point average (CGPA) not less than 1.00. GRADE POINT AVERAGE (GPA) AND CUMULATIVE GRADE POINT AVERAGE (CGPA) For each year of study, except in the first year of study when only the GPA will be used the CGPA of a student shall be calculated. Any student whose CGPA is less than 1.00 is placed on probation. If after one year (that is two consecutive semesters) the CGPA is still less than 1.00 the student shall be advised to withdraw from the Department. CLASSES OF DEGREE The classes of degree are divided as follows:4.50 – 5.00 First Class Honours 3.50 – 4.49 Second Class Honours (Upper Division) 2.40 – 3.49 Second Class Honours (Lower Division) 1.50 – 2.39 Third Class Honours 1.00 – 1.49 Pass EXAMINATION REGULATIONS AND CONDUCT OF STUDENTS AT EXAMINATIONS: Students shall: (a) Use or consult, during an examination, only such books, papers notes, instruments or other materials or aids as are specifically permitted or provided by the Department whose examination is being taken. (b) Not introduce nor attempt to introduce any books, papers notes, instruments or other materials or aids into the examination room. (c) Not pass or attempt to pass any information from one person to another during an examination. (d) Not Contact another person to write exam for him (impersonation) MISCONDUCT (a) Failure to observe any of the above rules shall, prima facie, constitute misconduct; (b) At the discretion of the Chief invigilator a candidate may be required to leave the examination room when his conduct is judged to be disturbing or likely to disturb the examination. The Chief Invigilator shall report any action taken to the Dean immediately. (c) Senate shall decide the penalty in cases of proven misconduct which may include (i) Suspension for one semester (ii) Suspension for one academic session and (iii) Expulsion from the University for beaching the provisions of examination. STUDENTS’ CHEMICAL SOCIETY OF NIGERIA (SCSN) All registered students in the department are members of the LAUTECH branch of the Students’ Chemical Society of Nigeria (SCSN). SCSN, LAUTECH Chapter is actively engaged in activities that promote the understanding of Chemistry and the physical environment as well as generating interest in the study of Chemistry among secondary schools students in the immediate area of the University. 6 WELFARE, GUIDANCE AND COUNSELING STAFF / STUDENTS FORUM In addition to the Faculty forum, the Department organizes a departmental staff/ students forum at least one every academic year. The Forum provides avenue for discussion, exchange of ideas and the opportunity for hearing cases and complaint. Opportunity for disseminating official information, rules and regulations especially those that relate directly to our affected students are also discussed. Students are normally encouraged in such forums to raise issues of significance. The policy is to be seen as part of the efforts of the administration of the University to bridge communication gaps and to encourage the much-needed better understanding between the University and its students. STUDENS’ LEVEL ADVISOR Each level has lecturer as registration officer who advises and deals with the computation of results of students and with the Head of the Department to advise, guide and counsel students when consulted. HEALTH MATTERS For all health matters, staff and students consult the LAUTECH Health Center on the campus. It is to be noted that only the certificate from the Director of Health Center is needed and accepted to back up absence from lectures, examination etc. If a student happens to be treated outside the University Clinic, he/she should submit his/her papers to the LAUTECH Health Center for authentication. SOURCES OF INFORMATION The University provides information to students through notice boards, announcements by staff at lecture halls and laboratories. Students make use of the notice boards regularly. If a student seeks any information, he/she consults his/her course lecturers, level advisor or the Head of the Department. In addition, the information is disseminated through the University Internet facilities at the website: www.lautech.edu.ng as well as the Faculty website: www.lautechfpas.net under the Department of Pure and Applied Chemistry. CODING OF COURSES A Chemistry course code normally comprises three digits: i. The first digit represents the level of the course, e.g. 1-100 level; 2-200 level etc. ii. The second digit represents the types of course, e.g. 0 - General; 1 - Inorganic; 2 – Organic 3 - Physical; 4 - Analytical; 5 - Industrial; 6 - Applied; 9 - Practical iii. The third digit denotes the semester in which the course is to be taught e.g. odd number - Harmattan Semester and even number for Rain Semester. 46. ACADEMIC CONTENT: (a) 46. (b) BACHELOR OF TECHNOLOGY: (B.TECH) PURE & APPLIED CHEMISTRY Philosophy, objectives and vision: Philosophy: The Department of Pure and Applied Chemistry’s philosophy is to provide its students with a broad based knowledge in theoretical and applied chemistry. At the end of the course, graduates would have understood the fundamental principles of chemistry and acquired enough practical experience to fit into chemical and allied industries or to be capable of being self - employed. 7 The undergraduate programmes are designed to be unique in content in the following respects: - - - To train students in Basic and Pure Chemistry that encompass all area and field of Chemistry including Physical, Organic, Inorganic, Industrial and Analytical Chemistry; To also train students as well in the following areas of applied chemistry: Theoretical Physical Chemistry, Biophysical Chemistry, Natural Product Chemistry, Organic Synthesis Chemistry, Geochemistry, Inorganic and Bioinorganic Chemistry, Environmental and Industrial Chemistry, Food Chemistry and Process Chemistry. To offer opportunities which encourage the advancement of learning and to hold out all persons without distinction or race, creed, sex or political conviction as well as the opportunities of acquiring a higher education in Pure and Applied Chemistry fields; Vision: We envision developing our research potentials to enviable level comparable to any novel research work in the aforementioned applied fields. We hope to produce the best crops of graduates that will provide work force in all facets of endeavours, in Nigeria or abroad. Objective: The objectives of the Department include: To develop and offer academic and professional programmes leading to the award of diploma, first degree, postgraduate research and higher degrees which emphasize planning adaptive, technical maintenance, developmental and productive skills in the engineering, scientific, agricultural, medical and allied professional disciplines with the aim of producing mature men and women with capability not only to understand, use and adapt existing technology, but also to improve thereon and develop new ones suited to the Nigerian Environment; To act as an agent for the effective and economic mobilization, excitation, conservation and development of the country’s natural economic and human resources through postgraduate training, research and innovation; To offer to the general population as a form of public service, the result of training and to foster the practical application of the results; . To establish appropriate relationship with other national and international institutions involved in training, research development and technology; To identify the technological problems of the society and to assist in finding solution to them within the context of overall national development; To research into indigenous technology so as to develop, modernize and relate them to the social, cultural, technological and economic needs of the people of Oyo and Osun States in particular and of Nigeria and the world in general. 8 To promote scholarship and advancement of Pure and Applied research in all field of learning and human endeavour. Of the ten minimum semesters required to be spent by each student, students are to spend six months during the eighth semester in industries or government/ private research laboratories working full time on any assigned projects or works involving application of Chemistry. During the fourth and fifth year, students are taught mainly Applied Chemistry courses which prepare them for future research work and to enable them acquire enough experience to fit into chemical and allied industries or to be capable of being self - employed. 46 (c) Admission Requirements: Candidates are admitted at two (2) entry points: 100 and 200 levels. For admission into 100 Level Candidates are admitted to the university through three (3) procedures as enumerated: UME CANDIDATES The minimum requirements for admission to courses leading to B.Tech. Degree in the Department of Pure and Applied Chemistry are those for entry into the Faculty of Pure and Applied Sciences. The University stipulates that candidates are required to have a minimum of credits in five (5) subjects at the SSCE or WASC or NECO level or GCE ‘O’ level including: Mathematics, Physics, Chemistry, Biology and English Language. This category of candidates is expected to meet the cut-off mark for such admission period. PRE-DEGREE CANDIDATES The admission requirements are as specified for the UME candidates above. Such candidates who have successfully gone through the University one year Pre-degree program are also expected to meet the specified minimum cut-off mark in the PDS examination before admission. For admission into 200 Level The admission to 200 Level is based on Direct Entry mode. DIRECT ENTRY CANDIDATES Candidates are required to have good passes at Advanced Level of GCE (or equivalent) in Physics, Chemistry, Mathematics, English Language, or approved equivalent qualifications: such as OND UPPER Credit. Such candidates are also expected to have minimum of credit pass in the five ‘O’ level science subjects as required by the Faculty and in line with the University policy. Inter/Intra School Admission This type of admission is into the 200 level. Candidates from recognized and reputable institutions may be allowed to transfer to 200 level provided they meet the relevant minimum admission requirements of the university as stated earlier and subject to satisfactory report not related to any disciplinary action from the destination university. Intra university candidates are required to fulfill university, faculty and departmental courses specifications for 100 level students. However, candidate transferring within the 9 university are expected to have passed all their 100 level courses before they can be considered (i.e. no Course Still Outstanding (CSO). They are also expected to meet the departmental requirements for such prevailing academic session. 46 (d) OUTLINE OF THE PROGRAMME CURRICULUM 100 LEVEL COURSES HARMATTAN SEMESTER Course Code Course Title Pre-requisite PHY 101 General Physics 1 PHY 103 Experimental Physics 1A CHM 101 General Chemistry l CHM 191 Experimental Chemistry 1 BIO 101 General Biology 1 BIO 103 Experimental Biology 1 MTH 101 Elementary Mathematics 1 GNS 101 Use of English FAA 101 Fundamental Drawing LIB 101 Use of Library Total Week L T P Load(Unit) 3 1 0 4 0 0 3 1 3 1 0 4 0 0 3 1 3 1 0 3 0 0 3 1 4 1 0 5 2 0 0 2 1 0 3 2 1 0 0 0 Total No of Units 23 RAIN SEMESTER Course Code Course Title Pre-requisite PHY 102 General Physics 11 PHY 104 Experimental Physics 1B CHM 102 General Chemistry l1 CHM 192 Experimental Chemistry 11 BIO 102 General Biology 11 BIO 104 Experimental Biology 11 MTH 102 Elementary Mathematics 11 GNS 102 Use of English 11 GNS 104 Science and Technology in Africa CSE 100 Introduction to Computer Total Week L T P Load (Unit) 3 1 0 4 0 0 0 1 3 1 0 4 0 0 3 1 3 1 0 3 0 0 3 1 4 1 0 5 2 0 0 2 2 0 0 2 1 0 0 1 Total No of Units 24 200 LEVEL COURSES HARMATTAN SEMESTER Course Code Course Title CHM 211 Basic Inorganic Chemistry CHM 231 Basic Physical Chemistry CHM 241 Principles of Analytical Chemistry CHM 291 STA 207 CSE 201 BIO 201 GNS 209 Experimental Physical/Inorganic Chemistry Statistics for Physical Sciences Basic Computer Programming Systematic Biology Elements of Administration 10 Pre-requisite CHM 102 CHM 101 CHM101 CHM102 CHM101 L 3 3 2 T 1 1 0 0 0 Total Week P Load (Unit) 0 4 0 4 0 2 3 3 1 0 2 0 3 1 0 0 2 0 0 Total No of Units 1 4 3 2 2 21 RAIN SEMESTER Course Code CHM 222 CHM 252 CHM 292 BCH 202 PHY 202 BIO 202 MTH 202 CSE 204 GNS 202 Course Title Pre-requisite Basic Organic Chemistry CHM 102 Introduction to Chemical Processes Experimental Organic Chemistry CHM 102 Cell and Molecular Biology Elementary Modern Physics Biological Techniques Elementary Differential Equations Introduction to Programming Applications Logic, Philosophy and Science Total Week L T P Load (Unit) 3 1 0 4 2 0 0 2 0 0 3 1 2 0 0 2 3 0 0 3 1 0 3 2 3 0 0 3 2 0 0 2 2 0 0 2 Total No of Units 21 300 LEVEL COURSES HARMATTAN SEMESTER Course Code Course Title Pre-requisite CHM 311 Structural and Main Group Chemistry CHM 211 CHM 321 Alicyclic, Heterocyclic and CHM 222 Aromatic Chemistry CHM 331 Thermodynamics CHM 231 CHM 341 Classical Methods of Analysis CHM 241 CHM 351 Introduction to Application of CHM 222 Chemical Principles CHM 353 Heat Transfer CHM 231 CHM 355 Introduction to Polymer Chemistry CHM 391 Experimental Inorganic/Analytical Chemistry L 3 3 T 1 1 Total Week P Unit 0 4 0 4 2 3 1 1 0 1 0 0 0 3 3 2 1 1 1 1 0 0 2 2 CHM 211 0 0 6 CHM 231 Total No of Units 2 22 RAIN SEMESTER Analytical Chemistry Option Course Code CHM 312 CHM 322 CHM 324 CHM 326 CHM 332 CHM 342 CHM 392 Course Title Transition Metal Chemistry Spectroscopic Methods Fundamental of Physical Organic Chemistry Macromolecules in Nature Chemical Kinetics Separation Methods Experimental Organic/ Industrial Chemistry Pre-requisite CHM 211 CHM 222 CHM 222 L 3 2 2 T 1 1 1 Total Week P Unit 0 4 0 3 0 3 CHM 222 BCH 202 CHM 231 CHM 241 CHM 292 1 1 0 2 1 2 0 1 1 0 0 0 6 2 3 2 Free Elective Total No of Units 11 2 21 RAIN SEMESTER Industrial Chemistry Option Course Code Course Title CHM 322 Spectroscopic Methods CHM 324 Fundamental of Physical Organic Chemistry CHM 332 Chemical Kinetics CHM 342 Separation Methods CHM 352 Unit Operation CHM 354 Petroleum Chemistry CHM 392 Experimental Organic/ Industrial Chemistry MGS 540 Technology, Policy and Law Free Elective T 1 1 Total Week P Unit 0 3 0 3 1 2 2 2 0 1 1 0 0 0 0 0 0 0 6 2 3 2 2 2 2 0 0 2 2 21 Pre-requisite L CHM 222 2 CHM 222 2 CHM 231 CHM 241 CHM 231 CHM 222 CHM 292 Total No of Units 400 LEVEL COURSES HARMATTAN SEMESTER Course Code Course Title CHM 401 (O) Nutritional Chemistry CHM 421 (C) Organic Reaction and Synthesis CHM 431 (A) Electrochemistry CHM 433 (C) Group Theory and the Quantum Mechanics of Molecules CHM 441 (C) Electro analytical and Spectroanalytical Method CHM 453 (O) Textile and Dyestuff Chemistry CHM 455 (O) Agricultural Chemistry and Agrochemicals CHM 457 (I) Surface Chemistry and Electrode Potential CHM 459 (O) Basic Concepts in Drug Design CHM 463 (C) Quality Control and Industrial Safety CHM 491 (C) Experimental and Instrumental Methods Pre-requisite CHM 222 CHM 324 CHM 321 CHM 331 CHM 331 CHM 311 CHM 241 CHM 341 CHM 222 CHM 322 L 1 2 T 1 2 Total Week P Unit 0 2 0 4 1 3 1 1 0 0 2 4 2 1 0 3 1 1 1 1 0 0 2 2 CHM 331 CHM 332 CHM 222 CHM351 1 1 0 2 1 1 1 1 0 0 2 2 0 0 6 Maximum Total No of Units 2 24 RAIN SEMESTER ITF Industrial Training (IT) 4 Credit Units An industrial training of 6 months is planned to provide practical experience in application of Chemistry in the industry. Assessment is partly by job performance and partly through the report written by the student. 12 500 LEVEL COURSES HARMATTAN SEMESTER Course Code CHM 591 Course Title Research Project *Restricted Electives *Free Elective L T P Total No of Units Total Week Unit 3 8 2 13 *Students are free to choose any eight units of restricted electives from the following courses as well as free elective from any other courses that are of high importance to Chemistry in the University. RESRICTED ELECTIVES Course Code Course Title Pre-requisite L T Total Week P Unit CHM 511 CHM 222 2 0 0 2 CHM 355 CHM 321 CHM 421 Momentum, mass and heat transfer CHM 353 CHM 433 Environmental Chemistry CHM 441 CHM 341 Food Chemistry CHM 401 Derivatization in Analytical Chemistry Chemistry and Technology of Fibers CHM 421 Chemical Technology CHM 351 Industrial Chemical Process Water and Wastewater Treatment CHM 341 CHM 441 2 2 0 0 0 0 2 2 2 0 0 2 2 0 0 2 2 2 0 0 0 0 2 2 2 2 2 2 0 0 0 0 0 0 0 0 2 2 2 2 CHM 521 CHM 523 CHM 533 CHM 541 CHM 545 CHM 547 CHM 551 CHM 553 CHM 555 CHM 561 Radiochemistry and Nuclear Chemistry Polymer Synthesis Chemistry of Dyestuff RAIN SEMESTER Course Code CHM 592 Course Title Research Project *Restricted Electives *Free Elective L T P Total No of Units Total Week Unit 3 8 2 13 *Students are free to choose any eight units of restricted electives from the following courses as well as free elective from any other courses in the University of high importance to Chemsitry. 13 RESRICTED ELECTIVES Course Code Course Title CHM 512 Catalysis L 2 T 0 Total Week P Unit 0 2 CHM 514 2 4 4 2 0 0 0 0 0 0 0 0 2 4 4 2 2 0 0 2 2 0 0 2 2 0 0 2 2 0 0 2 2 0 0 2 2 0 0 2 2 0 0 2 2 2 0 0 0 0 2 2 CHM 522 CHM 524 CHM 526 CHM 532 CHM 542 CHM 544 CHM 552 CHM 554 CHM 556 CHM 558 Pre-requisite CHM 211 CHM 312 Organometallic Chemistry CHM 211 CHM 311 CHM 312 Chemistry of Natural Products CHM 222 CHM 324 Petrochemistry and Petroleum CHM 222 Geochemistry CHM 354 Pesticide Chemistry CHM 459 CHM 455 Photochemistry CHM 222 CHM 231 Food Analysis CHM 401 BCH 201 Analysis of Selected Materials CHM 391 including Drugs CHM 392 CHM421 Chemistry and Technology of Dyeing CHM 222 And Pigmentation CHM 453 Wood, Pulp and Paper Chemistry CHM 231 CHM 453 Polymer Technology CHM 453 Mineral Processing CHM 211 14 46 (e) DESCRIPTION OF COURSES CHM 101: INTRODUCTORY CHEMISTRY I (4 Units) Fundamental chemical principles including detailed atomic structure and physical principles involves in chemical reactions is emphasized. Statistical treatment of data, measurement and precision, significant figures, methods of sciences, S.I. Units, theory of errors theory of sampling, nature theory. Atomic weight Avogadro’s number, structure of the atom, divisible atom, cathode rays, mass spectrometer, discovery of the nucleus, periodic law, electronic energy levels and the periodic table, atomic size, ionic atom potential, electron affinity, ionic radii, electronic configuration, molecular formulae, mole concept, calculations of formular and equations of gravimetric data and vice-versa, ionic equations of neutralization and precipitation. Concentrations, molarity and volumetric calculations based on stoichiometric coefficients, oxidations and reductions as electron balancing of redox equation by electron-transfer, equality; relevant calculations including volumetric analysis (e.g. KMnO4,etc) Chemical equilibra, the equilibrium state, mass action, equilibrium constant calculation, equilibrium changes, water equlibra and pH, weak acids, buffer solutions, dissociation reactions (using solubility products principles) as applied to qualitative and quantitative analysis, state of matter; balancing intermolecular forces, hydrogen bonding, order-disorder phenomena, entropy, free energy. Energy effects, exothermic and endothermic changes, enthalpy of reaction, Hess’s law of enthalpy summation, relevant calculation, heart of neutralization, combinations and formation, bond dissociation energies, relevant calculations, free energy and spontaneous change, electrochemistry; redox reactions, oxidation potentials treated in terms of free energy change, cells and batteries, introduction to chemical kinetics, basic definition of order of reaction, molecularity; reaction rates, activation energy, kinetic theory radioactivity. Pre-requisites: O-Level chemistry or equivalent. CHM 191 INTRODUCTORY EXPERIMENTAL CHEMISTRY I (1 Unit) A basic practical course to illustrate and demonstrate the principles of CHM 101. CHM 102: INTRODUCTORY CHEMISTRY II (4 Units ) Application of physical principles to descriptive chemistry of the elements and historical survey of the development and importance of organic chemistry. The molecule and chemical bonding; electrons in molecules, ionic bond, covalent bonds, polarity of bonds, coordinate bonds, metallic bonds, basic crystalline structures, e.g. NaCl and metallic lattices, periodic table: trends in properties of the elements, structures. Ionization energies, physical properties, chemical properties of elements, properties of selected types of compounds related to the periodic law-hydrides, oxides, acids and basis; properties of elements and their compounds, hydrogen, qualitative analysis, alkali metals (Na and K particularly); alkaline earth metals, d-block elements-complexes: chemistry of Cr, Fe, Co, Ni, Cu, particularly of the most common oxidations states, comparisons of transition elements of groups I and II with main group I and II, main group V trends, and chemistry of main group IV trends, main group V trends and Chemistry of N and P (particularly oxides), main group VI and chemistry of O, main group VII, main group VIII and the inert gases. Introduction to Organic Chemistry; scope of Organic chemistry and natural sources of organic compounds, with emphasis on coal and petroleum sources, hybridization and shapes of carbon compounds, characteristics of covalent bonds, bond length, bond angles and bond strength, and consequences on reactivity, polarity of bonds and molecule and consequences on reactivity, polarity of bonds and molecule and consequences on physical and chemical properties of organic compound, intermolecular forces and consequences on melting point; and boiling point of organic compound, acid-base behaviour of organic compounds, chemical principles and 15 methods of detection of elements in organic compounds; isolation and purification of organic compounds; determination of empirical and molecular and structural formulae of organic compounds, isomerism in organic compounds, principles of organic nomenclature with emphasis on IUPAC system for all functional groups; common types of organic reactions-addition, substitution, elimination, rearrangements of the common functional groups, structure and general reactions of alkanes, alkenes, alkynes, alcohols, aldehyde, acids, ketones, and amines; introduction to aromatic compounds, introduction to macro-molecular compounds and natural products. Pre-requisites: O-Level Chemistry or equivalent CHM 192: INTRODUCTION EXPERIMENTAL CHEMISTRY II A basic practical course to illustrate and demonstrate the principle of CHM 102 (1 Unit ) CHM 211: BASIC INORGANIC CHEMISTRY (4 Units ) Molecular orbital theory of simple Homonuclear and Heteronuclear diatomic molecule, introductory symmetry inorganic applications of standard reduction table. Introduction to complex ions, nomenclature and isomerism. Stereochemistry of compounds. Hard and soft acids and bases, reactions in aqueous and non-aqueous media. Detailed Chemistry of alkali and alkaline earth metals. First row transition elements (each elements from Ti-Cu should be discussed in terms of oxidations states, physical/chemical properties, compounds, complexes and biological significance). CHM 231: BASIC PHYSICAL CHEMISTRY (4 Units ) The aim of the course is to provide a solid and basic foundation in thermodynamics and kinetics treated under the following heading: kinetic theory: energies; the first law of thermodynamics; free energy; entropy and second law of thermodynamics; phase change; equilibrium; reaction kinetics; electrochemistry. Pre-requisites: CHM 101 or A-Level Chemistry and Maths or Physics Co-requites: Mathematics (1st year). CHM 241: PRINCIPLES OF ANALYTICAL CHEMISTRY: (2 Units) A course of lectures covering theoretical principles of analytical chemistry. The quantitative and statistical treatment of experimental data, theory of sampling, gravimetric methods, chemical methods of analysis; separation technique (chromatography, colour TLC, GC etc), physiochemical method, optical method, electrochemical methods (polarography, polarimetry, and ion-selective electrodes), radio-analytical techniques. Pre-requisites: CHM 101 and CHM 102 CHM 291: EXPERIMENTAL PHYSICAL/INORGANIC CHEMISTRY (1 Unit) A basic practical chemistry course designed to (a) Develop good laboratory practice (b) Illustrate the principles of the topic covered in the 200 level chemistry courses. (c) Demonstrate the empirical nature of chemistry. Basic techniques to be developed are in physical and inorganic chemistry, and shall include: (i) Estimation of errors, theoretical processing of experimental data (ii) Quantitative inorganic analysis by volumetric, gravimetric and optical methods including: (a) Measurement of pH and preparation of buffer solution (b) Oxidation-reduction titration (c) Mixed base titration requiring the use of more than one indicator, (i) Thermal analysis, including: (a) measurement of heat of reaction and (b) measurement of heat of solution and mixing (ii) analysis of intermolecular forces; (iii) chemical kinetics; (iv) electrochemistry (v) simple inorganic synthesis 16 Pre-requisites: CHM 191 CHM 222: BASIC ORGANIC CHEMISTRY (4 Units) Revision of chemistry of common functional groups covering the material of CHM 102. Extension of aliphatic chemistry, including hydrocarbons, alkenes, the carbonyl group, carboxylic acids and their derivatives. Survey of aromatic chemistry, topics include benzene and its mono-substituted products. Bifunctional compounds. Introduction to lipids, carbohydrates amino acids and proteins, and to chromatographic and spectroscopic methods of investigating organic structures. Synthesis of some organic compounds. Pre-requisites: CHM 102 or A-Level Chemistry CHM 252: BASIC PRINCIPLES OF CHEMICAL PROCESSES (2 Units) Synthetic pathway, synthesis tree; Raw materials utilization; chemical processing technology, Alternative processing routes/ Techniques; Economics of production, production cost, capital cost, Research and development cost; Management in processes, process, production and consumption analysis; case studies e.g. soap making, textile and ceramics, cosmetics, industrial chemicals, cements, brewery etc. Term paper to test the application of the course. CHM 292: EXPERIMENTAL ORGANIC CHEMISTRY (1 Unit) A course designed to illustrate the principles covered in the lecture course i.e. CHM 222. Topic include separation, purification and identification of organic compounds by solvent extraction, distillation, crystallization and chromatography followed by determination of physical constants, simple organic synthesis and qualitative analysis by chemical methods Pre-requisites: CHM 192 CHM 311: STRUCTURAL AND MAIN GROUP INORGANIC CHEMISTRY (4 Units) Structural topics covered in the course include structures of metals and ionic crystals of types AB1, AB2, AB2, A2. Introduction to diffraction methods: x-ray, neutron and electron diffraction, lattice and crystals defects, introduction to crystal growth; structure of covalent molecules and of complex ions of main group elements. Introduction to the structure of transition metal complexes. Chemistry of groups III-VIII are surveyed with particular attention to group trends, hydrides, pseudohalides, oxides, chalcogenides and oxy-anions. Pre-requisites: CHM 211 CHM 321: ALICYCLIC, AROMATIC AND HETEROCYCLIC CHEMISTRY (4 Units) A broad-based course in general organic chemistry advancing on CHM 222. Topics to be covered include: (a) Extension of aliphatic chemistry with emphasis on bifunctional compounds and discussion of some organosulphur and organophosphorus compounds. (b) Some features of alicyclic chemistry including important naturally occurring derivatives, naphthalene and anthracene derivatives, and polynuclear aromatic compounds. (c) Aromatic heterocycles exemplified by pyrole, furan, thiopene, pyridine, pyrene, indole, quinoline and benzopyrene systems. A brief account of non-aromatic heterocycles. (d) Biogenetic principles in natural product chemistry. Pre-requisites: CHM 222 CHM 331: THERMODYNAMICS (3 Units) The first, second and third law of thermodynamics are given a more rigorous treatment than CHM 231. thermodynamic principles are considered in relation to chemical potential, interrelationships of the thermodynamic functions; phase equilibria; gaseous and liquid mixture; Colligate properties of solution; chemical equilibria electrolyte solutions; thermodynamics of surface. Introduction to statistical thermodynamics. Pre-requisites: CHM 231 17 CHM 341: CLASSICAL METHODS OF ANALYSIS ( 3 Units) Practical fundamentals: Handling of reagents, apparatus, technique of common operation, volumetric application and it uses. Theory of acid base titrations Theory redox titration. Theory of precipitation titration. Theory of complexometric titration. This would include endpoint detection in each case. Gravimetric methods. Theory of titrations in non-aqueous media. Pre-requisites: CHM 241 CHM 351: INTRODUCTION TO APPLICATION OF CHEMICAL PRINCIPLES (2 Units) The purpose of the course is to demonstrate the independence of basic science and technology in the chemical and allied industries. Topics covered will include, basic chemical processes exemplified by the manufacture of dye intermediates, and detergents; synthetic fibres, plastics and resins, finishing agents, food preservatives and other selected industrial and fine chemicals, flow sheets, material and energy balance, pilot plants, models and scale-up principles, Optimization. Pre-requisites: CHM 222 CHM 391: EXPERIMENTAL INORGANIC/ ANALYTICAL CHEMISTRY (2 Units) Titration involving: Acid/based Reaction; Redox Reaction; Precipitation Reactions Complex formation Reaction; Gravimetric Analysis; Preparation and properties of simple inorganic complexes. Pre-requisites: CHM 291 CHM 312: TRANSITION METAL CHEMISTRY (4 Units) Preparation and characterization of coordination compounds. Brief historical survey of theories of bonding particularly CFT and MO theories. Introduction to electronic spectra complexes and magnetochemistry. Second and third row transition elements. Lanthanides and actinides. F-block chemistry is discussed in terms of electronic configuration, characteristics oxidation state, spectroscopy, magnetic properties, complex formation and separation processes Pre-requisites: CHM 211 CHM 322: SPECTROSCOPIC METHODS (3 Units) A survey of spectroscopic and optical methods with emphasis on their application in elucidation of structures of organic, inorganic and organ metallic compounds. Principles and applications of ultraviolet, infrared nuclear magnetic resonance and mass spectroscopy; optical rotation and optical rotatory dispersion. The complementary nature of spectroscopic and chemical methods of structure elucidation exemplified by applications in natural product chemistry. Spectroscopic of inorganic compounds and organometallic complexes. Tutorial work involves the interpretation of spectra. Pre-requisites: CHM 222 CHM 324: FUNDAMENTALS OF PHYSICAL ORGANIC CHEMISTRY (3 Units) Experimental methods for investigating reaction mechanisms; acid-base reaction; chemical kinetics and isotope effects; structure reactivity relationships aromaticity; hyperconjugation and tautomerism; classes and mechanisms of organic reactions, viz addition, substitution, elimination, rearrangements, reduction, oxidation, etc. Medium effects on organic reactions; stereochemistry and conformational analysis preparation of stereoisomerism, stereoselectivity, stereospecificity neighboring group effects. Pre-requisites: CHM 222 18 CHM 326: MACROMOLECULES IN NATURE (2 Units) The course is specifically designed to introduce students to macromolecules that occur naturally as a prelude to the more detailed study of macromolecule in the final year. Topics will include: macromolecules in nature, basic concepts, nomenclature and classification into typesnatural and artificial. Proteins nomenclature, separation and isolation of peptides; properties and structure of proteins, carbohydrate; nomenclature; classification and occurrence; reactions; configuration of monosaccharides; disaccharides and polysaccharides, lipids classification, triglycerides, structures, composition and function in living organisms; fatty acid and importance; properties of lipids; saponifications, halogenations, rancidity, et, commercial sues, soaps, detergents, edible fats, e.t.c.; phospholipids, Structure, composition, analytical methods for the characterization of fat and oils. Pre-requisites: CHM 22; BCH 202 CHM 332: CHEMICAL KINETICS (2 Units) Topics covered include: Review of reaction rates, rate equations, order of reactions and their determination, calculations; experimental methods for studying slow and fast reaction; theories of reaction rates; reactions in solution; complexes reactions; heterogeneous catalysis; free radical reaction mechanism; photochemical reaction mechanisms. Pre-requisites: CHM 231 CHM 342: SEPARATION METHODS (3 Units) Topics to be covered include General theory, instrumentation and applications of chromatographic methods, viz: liquids, Gas chromatographic methods, Solvent Extraction; Electrophoresis. Pre-requisites CHM 241 CHM 352: UNITS OPERATIONS (2 Units) Introductory fluid mechanics and fluid handling process; physicochemical industrial processes; grinding, size reduction; extraction, filtration, distillation and solvent extraction processes. Pre-requisites: CHM 231 CHM 354: PETROLEUM CHEMISTRY (2 Units) Composition, classification, and properties of petroleum and its products; processing of petroleum and hydrocarbons. Preparation and chemical transformation of primary petrochemicals. Pre-requisites: CHM 222 CHM 392: EXPERIMENTAL ORGANIC/INDUSTRIAL CHEMISTRY (2 Units) A more advanced course in experimental organic chemistry designed to provide experience in single and multi-stage synthesis, in separation and purification procedure, and in the identification of organic compounds with the aid of spectra in conjunction with chemical tests. The industrial applications should be taken into consideration in designing these experiments. The exercise is designed to involve deductive reasoning, based upon principles discussed in lectures. Pre-requisites: CHM 292 CHM 401: NUTRITIONAL CHEMISTRY (2 Units) This course is a chemical approach to food science, dealing mainly with the chemistry of the major constituents of food, their requirements and metabolism. Topics include: Food and its functions, type of nutrients, food as source of energy and use of energy by the body enzymes, classification, selectivity, sensitivity, digestion and absorption, stages of digestion and transport in the body; chemistry of oils, fats and colloids, rancidity, uses of emulsifying agents; dairy products, carbohydrates, foodstuffs, amino acids and proteins, protein requirements; water and mineral elements, water and solvent, trace elements, 19 vitamins; Food spoilage, food poisoning; chemicals in Food, chemical contaminants, food additives and flavoring agents. Pre-requisites: CHM 222 CHM 421: ORGANIC REACTIONS AND SYNTHESIS (2 Units) Selective types of reactions are discussed in relation to mechanistic concepts and to the utility of the process in modern organic chemical practice. Discussion include alkylation and acylation processes, aldol-type condensations, synthesis with organometallic compounds. Applications of these operations are exemplified by reference to synthesis in literature Pre-requisites: CHM 321 CHM 431: ELECTROCHEMISTRY (2 Units) Transport and thermodynamic properties of ionic solutions are considered; discussions include methods of measurement of conductance and of activity coefficients. Topics include electronic measurements of cell potential, electrical double layer, potential at zero charge, polarizable and non-polarizable interface, mass transport, concentration polarizable, Fick’s law, Levis equation, standard cell and their construction, voltametric techniques. Pre-requisites: CHM 331 CHM 433: GROUP THEORY AND THE QUANTUM MECHANICS OF MOLECULES (4 Units) An introduction to the method of quantum mechanics and their application to the study of structures of atoms. This is briefly considered in terms of historical development, the Schrodinger equation, application to simple systems like the hydrogen atom. (i) Group theory: basis theory of groups; symmetry elements and symmetry operations; molecular symmetry, point group; representation of groups, symmetry consideration in quantum mechanical calculations; applications of group theory in spectroscopy; symmetry classifications of vibrations of vibrational modes. (ii) Molecular orbitals: H2+ ion molecule and H2 molecule; molecular spectroscopic states, review of electronic configuration of homo and heteronuclear diatomic molecules spectroscopic states and correlation diagrams. (iii) Valence bond theory: H2 molecule; other simple diatomic molecules (Li2, N2, O2); introduction of ionic character into VB wave functions; comparison of MO and VB theories of diatomic molecules. (iv) Bonding in polyatomic molecules: hybridization and resonance MO and VB theories for polyatomic molecules. Pre-requisites: CHM 331 and 311 CHM 441: ELECTROANALYTICAL AND SPECTROANALYTICAL METHODS (3Units) Topics to be covered include: Electrogravimetry, coulometry, potentiometry, conductometric titrations, voltametry and Amperometry. Others are coulorimetry, spectrophotometry, flourimetry and flame spectrometry. Pre-requisites: CHM 341 CHM 453: TEXTILE AND DYESTUFF CHEMISTRY (2 Units) Introduction to the chemistry of textile fibers and dyestuffs, survey of natural and synthetic fibers. Classification of dyestuffs in relation to their applications. Physico-chemical characterization of dyes; applications of dyes to foods and drugs. Pre-requisites: CHM 222 20 CHM 455: AGRICULTURAL CHEMISTRY AND AGROCHEMICALS (2 Units) The chemistry of fertilizers, insecticides, fungicides, herbicides and growth regulators. Recent trends in the synthesis and structural elucidation of commercial fertilizers and pesticides. Pre-requisites: CHM 332 CHM 457: SURFACE CHEMISTRY AND ELECTRODE PROCESS (2 Units) Thermodynamics and electrical surface phenomena. Adsorption at the gas-solid, liquidgas and solid-solid interface, electrolytic conductance. Electrode process. Electrochemical cells. Thermodynamics and kinetics of electrode process. Pre-requisites: CHM 331 and CHM 332 CHM 459: BASIC CONCEPTS IN DRUG DESIGN Classification of various types of drugs. Chemistry and structures-activity relationships of drugs like sulfonamides, antibiotics, sulfones, antimalarials. Metabolism of drug in the body. Pre-requisites: CHM 222 CHM 463: QUALITY CONTROL AND INDUSTRIAL SAFETY (2 Units) Quality control as applied to selected products. Prevention and control of industrial and laboratory hazards. Pre-requisites: CHM 351 CHM 491: EXPERIMENTAL INSTRUMENTAL METHODS (2 Units) Practical classes to demonstrate instrumental methods in titration involving: atomic absorption spectrometry, UV/ Visible spectrophotometry, potentiometry and others. Pre-requisites: CHM 391 CHM 591/ 592 RESEARCH PROJECTS (6 Units) Projects will be on applications of chemistry in industries or other areas of science and technology. Each student shall write a project thesis and shall defend thesis orally before a Departmental Examining Panel. CHM 511: RADIOCHEMISTRY AND NUCLEAR CHEMISTRY (2 Units) Natural radiations, fusion, fission, decay processes, nature of radiation, nuclear models. Energetics of nuclear reaction. Principles and measurements of radioactivity. Applications of radioactivity. Radiation hazards. Pre-requisite: CHM 222 CHM 521: POLYMER SYNTHESIS (2 Units) Polymerization processes, Stereo-specific polymerization. Co-polymerization (including block and graft co-polymerizations). Phase Systems for polymerization. Industrially important thermoplastic and thermosetting polymers. Polymethemes. Rubbers elasticity. Mechanical properties of polymers. Analysis and testing of polymers. Degradation of polymers including thermal, UV oxidative degradations and methods and methods of stabilizing polymers. Use of thermal UV stabilizers and antioxidants to be discussed. Pre-requisite: CHM 355 CHM 523: CHEMISTRY OF DYESTUFF (2 Units) General chemistry of benzene, naphthalene and anthracene with particular reference to derivatives useful in the synthesis of dyestuffs and intermediates. Specific reference to selected heterocycles relevant to dyes and pigments, manufacture e.g. Pyrazolones, indole, thiazole etc. Pre-requisite: CHM 421 21 CHM 533: MOMENTUM, MASS AND HEAT TRANSFER (2 Units) Introduction to fluid mechanics. Principles of heat and mass transfer. Industrial applications of these processes. Pre-requisite: CHM 353 CHM 541: ENVIRONMENTAL CHEMISTRY Sources of pollution. Formation and control of air pollutants. Interaction of gaseous pollutants with materials at the surface of the earth. Organic chemical pollution – petroleum, pesticides, detergents, etc. Pollution through trace metals, nuclear pollution, water pollution, water desalinization. Noise as an environmental pollutants, thermal pollution. Pre-requisite: CHM 441 CHM 545: FOOD CHEMISTRY (2 Units) Occurrence, structures and functions of carbohydrate including starch, source, physical and chemical properties. Starch behavior during baking and stating of bread. Glucose syrup – chemistry and enzymatic productions. Peptic substance, nucleic acid and proteins. Fats and oil oils. Ripening and denaturation of fruits – physical and biochemical changes in fruit ripening. The chemistry of fermentation processes in food industry. Effect of enzyme in foods. Enzymic and non-enzymic brewing. Thermolytic degradation of fatty acids. Antioxidation of fatty acids. Food preservation techniques, water activity. Pre-requisite: CHM 401 CHM 547: DERIVATIZATION IN ANALYTICAL CHEMISTRY (2 Units) General discussions on the usefulness, theory and application of derivatization in analytical chemistry. CHM 551: CHEMISTRY AND TECHNOLOGY OF FIBERS (2 Units) Introductory survey of natural and artificial fibers. Chemistry of cotton wool, polyesters, polyamides (nylons), polyacrylonitrile and polyolefin fiber. Physical properties of textile fibers. Heat and moisture relations in textiles. A brief introduction to the mechanical process of converting fibers into yarns and fabrics. Pre-requisite: CHM 453 CHM 553: CHEMICAL TECHNOLOGY (2 Units) Detailed processing, physical principles, flow charts and chemical conversion routes. Batch and continuous processes. Basic industrial equipment. Chemical process selection design and operation. Elementary control and instrumentation. Chemical process economics. Introduction to the technology of selected industries in Nigeria. Comprehensive description of processing technologies of selected inorganic and organic based industries: coal, fuel/ industrial gases, ceramics, phosphorus, glass, soap, detergents, plastic, sugar, paint, varnishes, selected oils and fats. Indigenous technology e.g. soap making, dyeing, brewing, etc. Shortcomings, recommendations etc. Pre-requisite: CHM 351 CHM 555: INDUSTRIAL CHEMICAL PROCESS (2 Units) Production of primary intermediates and synthesis of industrial organic chemicals, insecticides, pesticides, explosive herbicides, flavoring reagents, thickeners and extenders, rubbers, pharmaceuticals. Fermentation process. CHM 561: WATER AND WASTEWATER TREATMENT (2 Units) Detailed water analysis. Sources of water pollutants, study of water effluent, water treatment with reference to specific industries. 22 Pre-requisite: CHM 441 CHM 512: CATALYSIS (2 Units) General principles of catalytic processes. Homogeneous and heterogeneous catalysis including acid-base catalysis and catalysis involving metal complexes. Heterogeneous catalysis on solid surface. Kinetics and mechanisms of catalytic processes. Industrial application of catalysis. Chemistry and structures of commercial catalysts. CHM 514: ORGANOMETALLIC CHEMISTRY (2 Units) Classification of organometallic compounds. Preparation, structures and reactions including abnormal behaviour of organometallic compounds. Synthetic utility of organometallic compounds- Generation of free radicals- free organometallic compounds. Pre-requisite: CHM 311 CHM 522: CHEMISTRY OF NATURAL PRODUCTS (2 Units) Chemistry of terpenoids steroids and alkaloids, antibiotics, flavonoids, prostaglandins, coumarins and chlorophylls. Other natural products of pharmaceutical importance. General methods of isolation, separation, purification and structural determnination of the natural products. Classifications, discussions and discussions of important members. Biogenesis. Pre-requisite: CHM 222, CHM 324 CHM 524: PETROCHEMISTRY AND PETROLEUM GEOCHEMISTRY (2 Units) Origin of petroleum. Exploration techniques Petroleum in the contemporary energy scene. Nature, classification and composition of crude petroleum and natural gases. Distribution of petroleum and natural gas resources (global and Nigeria). Fractionation. Chemistry of refining process. Characteristics and uses of refinery products. Economic aspects of crude petroleum. Chemical feed stocks for petro-chemical industry. Unit operations involved in the processing f petro-chemical feedstocks. Chemical conversions- alkylation, amination, halogenation etc. Manufacture and prospects for petrochemicals in Nigeria. Aspects of organic geochemistry. Pre-requisite: CHM 354 CHM 526: PESTICIDE CHEMISTRY (2 Units) A detailed account of the different classes of pesticides including metabolism, chemical and physical properties. Pesticide residue analysis. Pre-requisite: CHM 455 CHM 532: PHOTOCHEMISTRY (2 Units) Interaction of radiations with matter. Electronic excitation, selection rules, deactivation routes, sensitization, quenching, photofragmentation, oxidation, reduction, rearrangement, pericyclic reactions and molecular orbital symmetry. Pre-requisite: CHM 222, CHM 231 CHM 542: FOOD ANALYSIS (2 Units) Sampling, sample treatment of analysis. Proximate analysis. Mineral analysis. Fatty acid. Sugar. Vitamins. Toxicants (e.g. tannins, phytates, glycosymolates, cyanide, saponin, alkaloid, anthraquinones, glycosides etc). Oil values rancidity, nuclear tides and melcric acids, volatile acids, alcohol, etc. (i) Sugar and fruit products (ii) Milk and dairy products (iii) Fleshy foods (meat and fish products) (iv) Fermented products (beer, wine, vinegar) (v) Flour and confectionary products 23 (vi) oils Pre-requisite: CHM 401 CHM 544: ANALYSIS OF SELECTED MATERIALS INCLUDING DRUGS (2 Units) Various techniques in use for the analysis of wide materials. Analysis of environmental sample e.g. pesticide residue, hydrocarbon and air. Analysis of heavy metals contaminants. Organic functional groups and drugs analysis. Soil and geochemical analysis. Pre-requisite: CHM421 CHM 552: CHEMISTRY AND TECHNOLOGY OF DYEING AND PIGMENTATION (2 Units) Topics covered include physical chemistry essential to dyeing; adsorption/ absorption and desorption isotherms; thermodynamics treatment of equilibrium kinetics; diffusion of dyes in textile, and methods of determination of diffusion coefficients. Theory of dyeing and dyeing equipment for batch and continuous dyeing of fibres, textile printing by block, roller, and screen transferred techniques. Printing styles i.e. by direct, discharge and resist. Textile printing paste formulation, rheological and chemical properties, physical principles of dyestuff fixation, ageing, steaming, baking and curing processes. Chemistry and application to textile materials of phthalocyanine and inorganic colorimg matters. Measurement of colour. Colour difference measurement: matching of dyes fabrics, colur fastness test procedures, and the assessment of results. Dyes in colour photography. Quality control procedure and coloration in textile industry. Pre-requisite: CHM 453 CHM 554: WOOD, PULP AND PAPER CHEMISTRY (2 Units) Forest conservation, exploitation and a forestation. Species, anatomy, physical properties and classification of wood. Preparation of wood for pulping, physical and chemical methods of pulping. Detailed studies of the technology of pulp and paper manufacture. Special papers and structural boards. A survey of pulp industries in Nigeria. Pre-requisite: CHM 453 CHM 556: POLYMER TECHNOLOGY (2 Units) Large-scale industrial polymerization process. Polymer processing, injection, extrusion, compression and transfer. Moulding of thermoplastics, polymer additives, polymeric surface coatings and adhesives. Pre-requisite: CHM 355 CHM 558: MINERAL PROCESSING (2 Units) Physical and chemical properties involved in the extraction and refining of metals by hydro, pyro and electro-metallurgical techniques. Extraction of selected metals featuring processing of ores, purification processes of the ores and uses of metals. Corrosion and passivity. minerals Pre-requisite: CHM 211 24 S/N 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. PROJECTS OF GRADUATES IN THE PROGRAMME IN THE LAST THREE YEARS MATRIC NAMES OF AUTHOR TITLE YEAR SUPERVISOR NO 053922 ADENIJI, Ademola Chemical Investigation on the 2008/2009 Prof. E.T. Ayodele Aramide Leaves of Azadirachta indica 042271 OLAYIWOLA, Akeem Synthesis, Purification, 2008/2009 Dr. I.A. Bello Olusegun application and Determination of Thermodynamic Parameters of Dyes on Polyamide Fibers (Wool and Nylon) 043190 OYEWOLE, Oyewumi Effect of Radiations on the 2008/2009 Dr. O.M. Olabemiwo Oyeniran composition of crude oil (Bitumen) 043812 ADELEKE, Zainab Inhibition of aspergillius/ yeast 2008/2009 Dr. (Mrs.) F.E. Idowu growth in zobo drinks using Adelowo natural extracts 043021 OLADITI, Oladayo A. Literature Review on 2008/2009 Dr. (Miss) F.A. Photophysical properties of Adekunle complexes 043717 OJEABULU, Victoria Milk processing on Nigeria 2008/2009 Prof. O.O.P Faboya Ekaniyere white cheese 042316 OYEWOLA, Shina Kinetic, equilibrium and 2008/2009 Dr. O.S. Bello Jeremiah thermodynamic studies of the adsorption of Nickel ion on orange mesocarp 043457 ODENIYAN, Temitope Synthesis and Characterization 2008/2009 Dr. (Mrs.) M.A. Opeyemi of copper (II) complex of Oladipo sodium barbitone and its toxicological effects on living rats 042463 ADESOYE, Abiola Nitrate content of smoked fish 2008/2009 Prof. O.O.P Faboya Olapeju 043059 OLATINWO, Adedotun Structural and electronic 2008/2009 Dr. B. Semire Ibukun properties of thiophene-1,1dioxide oligomer: a semiempirical study 043990 OMONIRA, Iwapele Chemical Investigation on the 2008/2009 Prof. E.T. Ayodele Taiwo leaves of azadirachta indica 043139 OPARINDE, Oluranti Kinetic study of lead (II) 2008/2009 Prof. A.A. Olajire Esther adsorption on melon husk 032995 OGUNLADE, Temitope Kinetics and equilibrium studies 2007/2008 Dr. O.S. Bello Micheal of adsorption of copper ions from aqueous solution using spear grass. (Imperita cylindrical) 032785 OWOJORI, Kehinde Isolation of flavonoids from the 2007/2008 Prof. C.O. Fakunle Ibunkun leaf extracts of Acanthospermum hispidum DG. 032710 BELLO, Ishaq Oladele Isolation and characterization of 2007/2008 Prof. C.O. Fakunle some diterpenoids from the latex of Euphorbia lateriflora 033050 MURITALA, Sakirat Removal of chromium metal 2007/2008 Dr. O.S. Amuda Abimbola from industrial waste water using agricultural waste “melon husk” 033156 ADEKOLA, Olapeju Proximate composition and 2007/2008 Dr. (Mrs) M.O. Bello Aderinola mineral content of two species of date palm seeds (phoenix dactylifera and phoenix reclinate) 25 18. 033410 AJAYI, Oluwamodupe Omolara 19. 033256 SALAMI, Sukurat 20. 031411 OYEDEJI, Ajimoh Bolanle 21. 033220 22. 031356 ADENIRAN, Mary Olubukola AMUSAT, Mutiat Nike 23. 012470 ADEDAPO, Adedoyin Oluseyi 24. 012743 AJAYI, Ayooluwa.O 25. 012378 ADEYEYE, Ronke Janet 26. 013058 ADEDOYIN, Basirat Omotayo 27. 012310 TAIWO, Shakirat Wunmi 28. 012559 JIMOH, Mistura Olubunmi 29. 012346 AKOGUN, Oludare Joshua 30. 012470 ADEDAPO, Adedoyin Oluseyi Synthesis and characterization of Cu (II) complexes using benzoic acid hydrazine as ligand. Synthesis and characterization of nickel complexes of benzoic acid hydrazide. Synthesis of Unsymmetrical monosulfide of the type RSR’butyl benzyl sulfide Synthesis and characterization of Zn(pNBAH)2SO4 Semi-empirical study of molecular structure and electronic properties of chloromethyl-furan oligomers The effect of the ligand metachlorobenzoic acid hydrazide and its nickel (II) complexes on cholinesterase Waste water treatment using Natural adsorbent (chrysophyllum albidum) Effect of butyrylacetic Acid hydrazide and its nickel (II) complexes of hydrazides on cholinesterase Critical appraisal of PAHs ratios as indicator of PAHs source and composition in a farmland of a coal-mining site. The removal of heavy metals from waste water using natural absorbent (corn-cob) Preparation of activated charcoal from agricultural waste (sawdust) and its application in the adsorption of zinc ion from wastewater. Kinetic and equilibrium studies of adsorption of Ni2+ on oil-palm fibre The effect of the ligand metal chlorobenzoic acid hydrazide and its nickel (II) complexes on cholinesterase. 26 2007/2008 Dr. I.O. Adeoye 2007/2008 Dr. I.O. Adeoye 2007/2008 Prof. E.T. Ayodele 2007/2008 Prof. O.A. Odunola 2007/2008 Dr. B. Semire 2006/2007 Prof. O.A. Odunola 2006/2007 Dr. O.S. Amuda 2006/2007 Prof. O.A. Odunola 2006/2007 Dr. T.A. Adedosu 2006/2007 Dr. O.S. Amuda 2006/2007 Dr. O.S. Amuda 2006/2007 Dr. O.S. Amuda 2006/2007 Prof. O.A. Odunola 47. Rain Table 4: Programme/Sub-Discipline/Discipline Workload by Chemistry Students (Harmattan and Semesters) 100 Level (Harmattan Semester) Grouping Course Code Prerequisite Course Title FAA 101 General Studies Core/Compulsory Courses GNS 101 LIB 101 BIO 101 BIO 103 CHM 101 CHM 191 MTH 101 PHY 101 PHY 103 Elective Courses Total Week Load Contact Hours/Week Fundamental of Drawing Use of English I Use of Library - Lecture 2 Tutorial 0 - 2 0 0 0 0 0 2 0 General Biology I Experimental Biology I General Chemistry I Experimental Chemistry I Elementary Mathematics I General Physics I Experimental Physics IA - 3 0 3 0 1 0 1 0 0 3 0 3 3 1 4 1 - 4 1 0 5 - 3 0 1 0 0 3 4 1 - - - - - Practical 0 2 NONE 23 TOTAL 100 Level (Rain Semester) Course Code Total Week Load Contact Hours/Week Grouping Prerequisite Course Title GNS 102 GNS 104 General Studies CSE 100 BIO 102 BIO 104 Core/Compulsory Courses CHM 102 CHM 192 MTH 101 PHY 102 PHY 104 Elective Courses Use of English II Science and Technology in Africa Introduction to Computer General Biology II Experimental Biology II General Chemistry II Experimental Chemistry II Elementary Mathematical II General Physics II Experimental Physics IB - Lecture 2 2 Tutorial 0 0 Practical 0 3 2 2 - 1 0 0 1 - 3 1 1 0 0 3 3 1 - 3 0 1 0 0 3 4 1 - 4 1 0 5 - 3 0 1 0 0 3 4 1 - - - - - NONE 24 TOTAL 27 200 Level (Harmattan Semester) Course Code Prerequisite Grouping Course Title General Studies Core/ Compulsory Courses Lecture Tutorial Practical GNS 209 Elements of Administration - 2 0 0 2 CHM 211 CHM 231 CHM 241 Basic Inorganic Chemistry Basic Physical Chemistry Principles of Analytical Chemistry Experimental Physical/Inorganic Chemistry Statistics for Physical Sciences Basic Computer Programming Systematic Biology CHM 102 CHM 101 CHM101 3 3 2 1 1 0 0 0 0 4 4 2 CHM101 0 0 3 1 3 1 0 4 2 0 3 2 2 - 0 - 0 - 2 - CHM 291 STA 207 CSE 201 BIO 201 Elective Courses Total Week Load Contact Hours/Week NONE 21 TOTAL 200 Level (Rain Semester) Course Code Total Week Load Contact Hours/Week Grouping Prerequisite Course Title GNS 202 Logic, Philosophy and Science - 2 Tutoria l 0 CHM 222 CHM 252 Basic Organic Chemistry Introduction to Chemical Processes Experimental Organic Chemistry Cell and Molecular Biology Elementary Modern Physics Biological Techniques Elementary Differential Equations Introduction to Programming Applications CHM 102 3 2 1 0 0 0 4 2 CHM 102 0 0 3 1 2 3 1 3 0 0 0 0 0 0 3 0 2 3 2 3 2 0 0 2 - - - - General Studies Core/ Compulsory Courses CHM 292 BCH 202 PHY 202 BIO 202 MTH 202 CSE 204 Elective Courses NONE Lecture Practic al 0 2 21 TOTAL 28 300 Level (Harmattan Semester) Grouping Course Code Total Week Load Contact Hours/Week Pre-requisite Course Title General Studies CHM 321 CHM 331 CHM 341 CHM 351 CHM 353 CHM 355 CHM 391 Elective Courses Tutorial - - - - Practi cal - Structural and Main Group Chemistry Alicyclic, Heterocyclic and Aromatic Chemistry Thermodynamics Classical Methods of Analysis Introduction to Application of Chemical Principles Heat Transfer Introduction to Polymer Chemistry Experimental Inorganic/Analytical Chemistry CHM 211 3 1 0 4 CHM 222 3 1 0 4 CHM 231 CHM 241 2 3 1 0 0 0 3 3 CHM 222 1 1 0 2 CHM 231 - 1 1 1 1 0 0 2 2 CHM 211 0 0 6 2 - - - - - - NONE CHM 311 Core/ Compulsory Courses Lecture NONE 22 TOTAL 300 Level (Rain Semester) Analytical Chemistry Option Grouping Course Code Total Week Load Contact Hours/Week Pre-requisite Course Title General Studies Core/ Compulsory Courses NONE CHM 312 CHM 322 CHM 324 CHM 326 CHM 332 CHM 342 CHM 392 Elective Courses - - Lecture - Tutorial - Practical - - Transition Metal Chemistry Spectroscopic Methods CHM 211 3 1 0 4 CHM 222 2 1 0 3 Fundamental of Physical Organic Chemistry Macromolecules in Nature Chemical Kinetics Separation Methods Experimental Organic/ Industrial CHM 222 2 1 0 3 CHM 222 BCH 202 CHM 231 CHM 241 CHM 292 1 1 0 2 1 2 0 1 1 0 0 0 6 2 3 2 2 0 0 2 FREE 21 TOTAL 29 300 Level (Rain Semester) Industrial Chemistry Option Grouping Course Code Total Week Load Contact Hours/Week Pre-requisite Course Title General Studies Core/ Compulsory Courses MGS 540 CHM 322 CHM 324 CHM 332 CHM 342 CHM 352 CHM 354 CHM 392 Technology, Policy and Law Spectroscopic Methods Fundamental of Physical Organic Chemistry Chemical Kinetics Separation Methods Units Operations Petroleum Chemistry Experimental Organic/ Industrial Chemistry - Lecture 2 Tutorial 0 Practical 0 2 CHM 222 CHM 222 2 2 1 1 0 0 3 3 CHM 231 CHM 241 CHM 231 CHM 222 CHM 292 1 2 2 2 0 1 1 0 0 0 0 0 0 0 6 2 3 2 2 2 2 0 0 2 Elective Courses - 21 TOTAL 30 400 Level (Harmattan Semester) Course Code Total Week Load Contact Hours/Week Grouping Prerequisite Course Title NONE - - Lectur e - Tutorial CHM 401 (O) CHM 421 (C) Nutritional Chemistry Organic Reaction and Synthesis Electro-chemistry Group Theory and the Quantum Mechanics of Molecules Electro analytical and Spectro-analytical Method Textile and Dyestuff Chemistry Agricultural Chemistry and Agrochemicals Surface Chemistry and Electrode Processes Basic Concepts in Drug Design Quality Control and Industrial Safety Experimental and Instrumental Methods CHM 222 CHM 324 1 2 1 2 0 0 2 4 CHM 331 CHM 311 CHM 331 1 3 1 1 0 0 2 4 CHM 241 CHM 341 2 1 0 3 CHM 222 1 1 0 2 CHM 322 1 1 0 2 CHM 331 1 1 0 2 CHM 222 1 1 0 2 CHM351 1 1 0 2 - 0 0 6 2 - - - - - - Practic al - - General Studies Core/ Compulsory Courses CHM 431 (A) CHM 433 (C) CHM 441 (C) CHM 453 (O) CHM 455 (O) CHM 457 (I) CHM 459 (O) CHM 463 (C) CHM 491 (C) Elective Courses NONE 23 Compulsory courses -15 units Restricted Electives - 8 units MAXIMUM TOTAL 400 Level (Rain Semester) Course Code Total Week Load Contact Hours/Week Grouping Pre-requisite Course Title General Studies Lecture Tutorial Practical INDUSTRIAL TRAINING (IT) ITF 4 Core/ Compulsory Courses Elective Courses - - - - NONE 4 TOTAL 31 500 Level (Harmattan Semester) Grouping Course Code NONE Course Title Prerequisite - Contact Hours/Week Total Week Load - Lecture - Tutorial - Practical - - 0 0 12 3 - General Studies Core/ Compulsory Courses Elective Courses CHM 591 Research Project CHM 511 Radiochemistry and Nuclear Chemistry CHM 222 2 0 0 2 CHM 521 Polymer Synthesis CHM 355 2 0 0 2 CHM 523 Chemistry of Dyestuff CHM 321 CHM 421 2 0 0 2 CHM 533 Momentum, mass and heat transfer CHM 353 CHM 433 2 0 0 2 CHM 541 Environmental Chemistry CHM 441 CHM 341 2 0 0 2 CHM 545 CHM 547 Food Chemistry Derivatization in Analytical Chemistry CHM 401 2 0 0 2 CHM 551 Chemistry and Technology of Fibers CHM 421 2 0 0 2 CHM 553 CHM 555 Chemical Technology Industrial Chemical Process CHM 351 - 2 2 0 0 0 0 2 2 CHM 561 Water and Wastewater Treatment CHM 341 CHM 441 2 0 0 2 Free MINIMUM TOTAL 32 Core courses Restricted Electives Free Elective - 3 units - 8 units - 2 units 2 13 500 Level (Rain Semester) Grouping Course Code Course Title NONE Prerequisite - Contact Hours/Week Total Week Load - Lecture - Tutorial - Practical - - 0 0 12 3 - General Studies Core/ Compulsory Courses Elective Courses CHM 592 Research Project CHM 512 Catalysis CHM 211 CHM 312 2 0 0 2 CHM 514 Organometallic Chemistry 2 0 0 2 CHM 522 Chemistry of Natural Products CHM 211 CHM 311 CHM312 CHM 222 CHM 324 2 0 0 2 CHM 524 CHM 222 CHM 354 2 0 0 2 CHM 526 Petrochemistry and Petroleum Geochemistry Pesticide Chemistry CHM 455 CHM 459 2 0 0 2 CHM 532 Photochemistry CHM 231 CHM 222 2 0 0 2 CHM 542 Food Analysis BCH 201 CHM 401 2 0 0 2 CHM 544 Analysis of Selected Materials including Drugs Chemistry and Technology of Dyeing and Pigmentation Wood, Pulp and Paper Chemistry CHM 391 CHM 392 CHM421 CHM 222 CHM 453 2 0 0 2 2 0 0 2 CHM 231 CHM 453 2 0 0 2 Polymer Technology Mineral Processing CHM 453 CHM 211 2 2 0 0 0 0 2 2 2 13 CHM 552 CHM 554 CHM 556 CHM 558 Free MINIMUM TOTAL 33 Core courses Restricted Electives Free Elective - 3 units - 8 units - 2 units 48. Table 5: Programme/Sub-Discipline/Discipline Workload by Staff 100 Level :Harmattan Semester Grouping Course Code No of Students Taught Staff Contact Hours Course Title General Studies Core/Compul sory Courses Elective Courses FAA 101 - GNS 101 LIB 101 - BIO 101 BIO 103 CHM 101 CHM 191 - MTH 101 - PHY 101 PHY 103 - Prerequisite Weekly Contact Fundamental of Drawing Use of English I Use of Library - Lectur e 2 Tuto rial 0 Practi cal 0 - 2 0 0 0 0 0 2 0 General Biology I Experimental Biology I General Chemistry I Experimental Chemistry I Elementary Mathematics I General Physics I Experimental Physics - 3 0 3 0 1 0 1 0 0 3 0 3 3 1 4 1 - 4 1 0 5 - 3 0 - 1 0 - 0 3 - 4 1 - Hours 2 NONE 23 TOTAL 100 Level (Rain Semester) Grouping Course Code No of Students Taught Staff Contact Hours Course Titles General Studies Core/Compul sory Courses Elective Courses GNS 102 GNS 104 - CSE 100 - BIO 102 BIO 104 _ - CHM 102 CHM 192 - MTH 102 - PHY 102 PHY 104 - Prerequisite Lecture Weekly Contact Hours Use of English II Science and Technology in Africa Introduction to Computer - 2 2 Tut ori al 0 0 - 1 0 0 1 General Biology II Experimental Biology II General Chemistry II Experimental Chemistry II Elementary Mathematics II General Physics II Experimental Physics IB - 3 1 1 0 0 3 3 1 - 3 0 1 0 0 3 4 1 - 4 1 0 5 - 3 0 1 0 0 0 4 1 - - - - - Practi cal 0 3 2 2 NONE 24 TOTAL 34 200 Level (Harmattan Semester) Course Code Grouping No of Students Taught Course Title General Studies Core/ Compulsory Courses Elective Courses GNS 209 CHM 211 150 CHM 231 150 CHM 241 150 CHM 291 150 STA 207 - CSE 201 - BIO 201 - Total Week Load Staff Contact Hours Elements of Administration Basic Inorganic Chemistry Basic Physical Chemistry Principles of Analytical Chemistry Experimental Physical/Inorganic Chemistry Statistics for Physical Sciences Basic Computer Programming Systematic Biology Prerequisite Lecture Tutorial Practical CHM 102 2 3 0 1 0 0 2 4 CHM 101 3 1 0 4 CHM101 2 0 0 2 CHM101 0 0 3 1 - 3 1 0 4 - 3 0 0 3 - 2 0 0 2 - - - - - NONE TOTAL 22 200 Level (Rain Semester) Grouping General Studies Core/ Compulsory Courses Elective Courses Course Code No of Students Taught GNS 202 CHM 222 150 CHM 252 150 CHM 292 150 BCH 202 - PHY 202 - BIO 202 - MTH 202 - CSE 204 - NONE - Staff Contact Hours Course Title Prerequisite Logic, Philosophy and Science Basic Organic Chemistry Introduction to Chemical Processes Experimental Organic Chemistry Cell and Molecular Biology Elementary Modern Physics Biological Techniques Elementary Differential Equations Introduction to Programming Applications - - - TOTAL Total Week Load Lecture 2 Tutorial 0 Practical 0 3 1 0 4 2 0 0 2 0 0 3 1 2 0 0 2 2 0 0 2 1 0 3 2 2 0 0 2 2 0 0 2 CHM 102 CHM 102 - - - 2 21 35 300 Level (Harmattan Semester) No of Grouping Course Students Code Course Title General Studies NONE CHM 311 CHM 321 Core/ Compulsory Courses CHM 331 CHM 341 CHM 351 CHM 353 CHM 355 CHM 391 Elective Courses Staff Contact Hours Lecture Tutorial Practical - - - CHM 211 CHM 222 3 1 0 4 3 1 0 4 CHM 231 CHM 241 CHM 222 2 1 0 3 3 0 0 3 1 1 0 2 CHM 231 - 1 1 0 2 1 1 0 2 CHM 211 0 0 6 2 Structural and Main Group Chemistry Alicyclic, Heterocyclic and Aromatic Chemistry Thermodynamics Classical Methods of Analysis Introduction to Application of Chemical Principles Heat Transfer Introduction to Polymer Chemistry Experimental Inorganic/Analytical Chemistry NONE - - - - - TOTAL 300 Level (Rain Semester) Analytical Chemistry Option No of Grouping Course Students Code General Studies Core/ Compulsory Courses Total Week Load - Prerequisite - 22 Staff Contact Hours Course Title Prerequisite Lecture Tutorial Practical - - - Total Week Load - NONE - - CHM 312 Transition Metal Chemistry Spectroscopic Methods Fundamental of Physical Organic Chemistry Macromolecules in Nature Chemical Kinetics Separation Methods Experimental Organic/ Industrial Chemistry CHM 211 3 1 0 4 CHM 222 2 1 0 3 CHM 222 2 1 0 3 CHM 222 BCH 202 CHM 231 1 1 0 2 1 1 0 2 CHM 241 2 1 0 3 CHM 292 0 0 6 2 CHM 322 CHM 324 CHM 326 CHM 332 CHM 342 CHM 392 Elective Courses TOTAL 2 0 0 2 21 36 300 Level (Rain Semester) Industrial Chemistry Option Grouping Course Code No of Students Prerequisite General Studies MGS 540 Core/ Compulsory Courses CHM 322 CHM 324 CHM 332 CHM 342 CHM 352 CHM 354 CHM 392 Total Week Load Staff Contact Hours Course Title Technology, Policy and Law Spectroscopic Methods Fundamental of Physical Organic Chemistry Chemical Kinetics Separation Methods Unit Operation Petroleum Chemistry Experimental Organic/ Industrial Chemistry Elective Courses TOTAL - Lecture 2 Tutorial 0 Practical 0 2 CHM 222 2 1 0 3 CHM 222 2 1 0 3 CHM 231 1 1 0 2 CHM 241 2 1 0 3 CHM 231 2 0 0 2 CHM 222 2 0 0 2 CHM 292 0 0 6 2 - 2 0 0 2 21 37 400 Level (Harmattan Semester) Grouping Course Code No of Students Staff Contact Hours Course Title - - - Total Week Load - CHM 222 1 1 0 2 CHM 324 2 2 0 4 CHM 331 1 1 0 2 CHM 311 CHM 331 3 1 0 4 CHM 241 CHM 341 2 1 0 3 CHM 222 1 1 0 2 CHM 322 1 1 0 2 CHM 331 1 1 0 2 CHM 222 1 1 0 2 CHM351 1 1 0 2 - 0 0 6 2 Prerequisite - NONE - CHM 401 (O) CHM 421 (C) CHM 431 (A) CHM 433 (C) Nutritional Chemistry Organic Reaction and Synthesis Electro-chemistry Group Theory and the Quantum Mechanics of Molecules Electro analytical and Spectroanalytical Method Textile and Dyestuff Chemistry Agricultural Chemistry and Agrochemicals Surface Chemistry and Electrode Processes Basic Concepts in Drug Design Quality Control and Industrial Safety Experimental and Instrumental Methods Lecture Tutorial Practical General Studies Core/ Compulsor y Courses CHM 441 (C) CHM 453 (O) CHM 455 (O) CHM 457 (I) CHM 459 (O) CHM 463 (C) CHM 491 (C) Elective Courses NONE MAXIMUM TOTAL - - - 23 Compulsory courses -15 units Restricted Electives - 8 units 400 Level (Rain Semester) Grouping General Studies Core/ Compulsory Courses Elective Courses Course Code No of Students Staff Contact Hours Course Title Prerequisite Lecture Tutorial Total Week Load Practical INDUSTRIAL TRAINING (IT) ITF 4 NONE TOTAL - - - 4 38 500 Level (Harmattan Semester) Grouping General Studies Core/ Compulsory Courses Course Code Course Title NONE - CHM 591 Research Project CHM 511 CHM 521 CHM 523 Elective Courses No of Students CHM 533 CHM 541 CHM 545 CHM 547 CHM 551 CHM 553 CHM 555 CHM 561 Prerequisite Staff Contact Hours Lectu Tutorial Practical re - - - - Total Week Load - - 0 0 12 3 Radiochemistry and Nuclear Chemistry Polymer Synthesis Chemistry of Dyestuff CHM 222 2 0 0 2 CHM 355 2 0 0 2 CHM 321 CHM 421 2 0 0 2 Momentum, mass and heat transfer Environmental Chemistry CHM 353 CHM 433 2 0 0 2 CHM 441 CHM 341 2 0 0 2 Food Chemistry Derivatization in Analytical Chemistry Chemistry and Technology of Fibers Chemical Technology Industrial Chemical Process Water and Wastewater Treatment Free CHM 401 2 0 0 2 CHM 421 2 0 0 2 CHM 351 2 0 0 2 - 2 0 0 2 CHM 341 CHM 441 2 0 0 2 Core courses - 3 units Restricted Electives - 8 units Free Elective - 2 units MINIMUM TOTAL 39 2 13 500 Level (Rain Semester) Grouping Course Code General Studies Core/ Compulsory Courses Course Title NONE - CHM 592 Research Project Catalysis CHM 512 Elective Courses No of Students Organometallic Chemistry CHM 522 Chemistry of Natural Products Petrochemistry and Petroleum Geochemistry Pesticide Chemistry CHM 526 Staff Contact Hours Lectu Tutorial Practica re l - - - CHM 514 CHM 524 Prerequisite - - Total Week Load - 0 0 12 3 CHM 211 CHM 312 2 0 0 2 CHM 211 CHM 311 CHM312 CHM 222 CHM 324 2 0 0 2 2 0 0 2 CHM 222 CHM 354 2 0 0 2 CHM 455 CHM 459 2 0 0 2 CHM 532 Photochemistry CHM 231 CHM 222 2 0 0 2 CHM 542 Food Analysis BCH 201 CHM 401 2 0 0 2 CHM 544 Analysis of Selected Materials including Drugs Chemistry and Technology of Dyeing and Pigmentation Wood, Pulp and Paper Chemistry Polymer Technology Mineral Processing CHM 391 CHM 392 CHM421 2 0 0 2 CHM 222 CHM 453 2 0 0 2 CHM 231 CHM 453 2 0 0 2 CHM 453 2 0 0 2 CHM 211 2 0 0 2 CHM 552 CHM 554 CHM 556 CHM 558 Free MINIMUM TOTAL Core courses - 3 units Restricted Electives - 8 units Free Elective - 2 units 40 2 13 49. Table 6: Teaching Staff Turnover: Summary of Teaching staff Turnover for the Programme/SubDiscipline/Discipline to be accredited. Staff Category/Designation No on Payroll Salary Scale/Step No. of Resignations or Dismissals in the Preceding Three years NIL Professor 7 CONUAS 07/06 Reader/Associate Prof. 6 CONUAS 06/10 NIL NIL Senior Lecturer 10 CONUAS 05/06 NIL NIL Lecturer I 3 CONUAS 04/10 NIL NIL Lecturer II 4 CONUAS 03/01 NIL NIL Assistant Lecturer 3 CONUAS 02/06 NIL NIL Graduate Assistant 2 CONUAS 01/01 NIL NIL NOTE: The Teaching Staff Turnover: Full Time Equivalent Rank Full Time Adjunct/PT Professor Reader Senior Lecturer Lecturer I Lecturer II Assistant Lecturer Graduate Assistant 5 6 8 3 0 1 2 TOTAL 2 2 0 4 2 - 41 Reasons for Resignation or Dismissal NIL Full Time Equivalent 7 6 10 3 4 3 2 32 50. Table 7: Personal Data for Staff Teaching All Courses of the Programme/Sub-Discipline/Discipline to be accredited. Supply the information in the table. Use additional sheets with the headings given below: Note: Take 3 hours of laboratory/Clinical Practical as 1 lecture full time (F/T) S/N 1. NAME OF STAFF 1 Dr. I. O. Adeoye RANK/DESIGN, SALARY SCALE, DATE OF FIRST APPOINTMEN T 2 Reader CONUASS 06 step 04 23/10/1991 F/T 3 F/T QUALIFICATION, DATE OBTAINED AND SPECIALIZATION, MEMBERSHIP OF PROFESSIONAL ASSOCIATION AND NUMBER OF PUBLICATIONS 4 B.Sc. (Hons.) Chemistry 1985, M.Sc. Inorganic Chemistry 1987, Ph.D. Inorganic Chemistry 2003. CSN, ICCON 23 Publications POST QUALIFICATION WORKING/TEACHING EXPERIENCE AND DATE, POST HELD AND THE ORGANISATION 5 Quay Staff, Nigerian Ports Authority Apapa July – December 1981 Teaching: NKST Secondary School Mkar-Gboko 1985 – 1986. Teaching: Tutorial College Lagos Limited, Lagos Jan – Dec. 1989. Teaching and Research: FedPoly, Ado-Ekiti 1990 – 1991. Teaching and Research: LAUTECH 1991-date 42 COURSE/ SUBJECTS TAUGHT 6 CHM 211, 311, 312 TEACHING LOAD/ LECTURE HOURS/ WEEK OTHER RESPONSIBILITIES/ INTEREST IN CURRICULAR AND EXTRA CURRICULAR ACTIVITIES 7 8 Current Ag. Head of Department. Former Ag. Head, Department of Science Laboratory Technology LAUTECH Member, Departmental Board of Examiners, Faculty Board of Examiners, Departmental Review Committee, Department al Examination Committee , Departmental Examination and TimeTable Committee, Chairman, Departmental Examination Administration, Departmental Registration Committee; Coordinator, Departmental Industrial Training, Member, 12/12 Senate, LAUTECH, Former Acting Head, Department of Chemical Sciences-Bells University of Technology, Ota, 2. Prof. O.O.P. Faboya Professor CONUASS 07 step 10 01/08/1991 F/T B.Sc. (Hons.) Chemistry 1970, Ph.D. Chemistry 1977, M.Sc. Food Science 1982, Diploma in Computer Studies 1994 CSN, NIFST, NIPA 35 Publications Teaching and Research: University of Ibadan 1979 to date Teaching and Research: LAUTECH 1991 to date CHM 341, 441, 545, 542, 602, 741, 748 8/8 3. Prof. O. A. Odunola Professor CONUASS 07 step 09 26/09/1991 F/T B.Sc. (Hons.) Chemistry 1984, M.Sc. Inorganic Chemistry 1986, Ph.D. Inorganic Chemistry 1989, Professional Qualification of Specialist in Polymer Science 1993 CSN, ACS, SAN, AAAS 40 Publications Teaching and Research: University of Ibadan 1986 to date Teaching and Research: LAUTECH 1991 to date Teaching and Research: OSU, Ago-Iwoye 1990-1991 Teaching and Research: University of Ilorin 19941995 CHM 211, 311, 312 6/6 43 Former Head, P/A Chemistry Department LAUTECH. Chairman, Examination Malpractices Committee, Member, University Senate Member, University Development Committee, Chairman, Management Board of LAUTECH Consultancy Services Member, 2007 NUC Programmes in Nigerian Universities Former Rector, The Polytechnic, Ibadan Former Dean Faculty of P/A Sciences LAUTECH, Member, University Senate Member, Faculty Board of Examiners Member, University Endowment Fund Former Exam Officer, Department of P/A Chemistry, LAUTECH 4. Prof. N.O. Olawore Professor CONUASS 07 step 09 /01/1991 F/T B.Sc. (Hons.) Chemistry 1974, M.Sc. Organic Chemistry 1976, Ph.D. Organic Chemistry 1985 CSN, ICCON 46 Publications Teaching: Molusi College, Ijebu-Igbo 1971-1974, Teaching and Research: The Polytechnic, Ibadan 1974 to 1980 Teaching and Research: Federal Polytechnic, Ilaro 1980-1990 Teaching and Research: LAUTECH 1991 to date CHM 222, 322, 324, 421, 522, 523, 624, 701, 732, 721, 722 12/12 5. Prof. A.A. Olajire Professor CONUASS 07 step 08 /09/1990 F/T B.Sc. (Hons.) Chemistry 1985, M.Sc. Industrial Chemistry 1988, Ph.D. Petroleum Chemistry 1996. CSN, NAPE, NYAS, SIGNCC 45 Publications Research Assistant: University of Ife 1989-1990, Teaching and Research: LAUTECH 1990-date Director, Acad. Planing CHM 351, 354, 541, 561, 512, 524, 544, 625, 634, 754, 752 12/12 6. Prof. E.T. Ayodele Professor CONUASS 07 step 08 01/10/1990 F/T B.Sc. (Hons.) Chemistry 1981, M.Sc. Organic Chemistry 1987, Ph.D. Organic Chemistry 1994. Teaching and Research: University of Ife 1983-1990, Teaching and Research: LAUTECH 1990-date Visiting Lecturer, University CHM 222, 321, 322, 324, 421, 455, 532, 701, 744. 44 12/12 First Head of Department of Science Laboratory Technology, FedPoly Ilaro, Former Acting Head, Department of P/A Chemistry, Member of Senate, Member of the Curriculum Committee of the Senate, Former, Commissioner for Education, Oyo State, Former Commissioner for Industry, Applied Science and Technology, Oyo State Former Ag. Head, P/A Chemistry Department LAUTECH. Managing Editor, Science Focus, An International Journal of Biological and Physical Sciences, Member, University Governing Council, LAUTECH, Member, University Senate, Faculty Board of Studies, Faculty Postgraduate Committee, Faculty Review Committee, Board of Pre-degree Science Programme, Examination Officer, External Examiner-FUTA and OAU. Dean, Faculty of P/A Science, Former Ag. Head, P/A Chemistry Department LAUTECH. Member, University CSN 27 Publications 7. Prof. J. Ige Professor CONUASS 07 step 10 14/09/1969 Adjunct B.Sc. (Hons.) Chemistry 1969, M.A. Chemistry 1971, Ph.D. Physical Chemistry 1974. SAN, CSN 36 Publications 8. Prof G. A. Olatunji Professor Sabbatic al B. Sc (Hons) Chemistry M. Sc., Ph. D. 9. Prof. (Mrs.) F.E. Adelowo Professor/ CONUASS 07 step 1 02/05/1991 F/T B.Sc. (Hons.) Chemistry 1977, M.Sc. Analytical Chemistry 1983, Ph.D. Chemistry 2002. CSN,NAWACS,TWO WS 24 Publications of North London, 1994 – 1995 Postdoctoral Fellows, Indian Institute of Chemical Technology, Hyderabad, India, 1997 – 1998. DAAD Short Visit Fellow, University of Duisburg, Germany, May – July 2002; University of Regensburg, Germany, August – September 2006; University of Regensburg, Germany, August – September 2009. Teaching And Research: OAU 1969-Date Teaching And Research: LAUTECH 2000-Date Teaching Assistant: Brandeis University, Massachusetts, USA 1972-1974 Senior Research: Dow Chemical Company, USA 1974-1975 Teaching and Research: LAUTECH 1991-date 45 Senate. Member, Faculty Board of Studies Member, Faculty Postgraduate Committee Member, Faculty Review Committee Member, Faculty Board of Pure and Applied Sciences Member, Board of Predegree Science Programme. CHM 331, 332, 433 4/4 CHM 322, 421, 522 4/4 CHM 341, 441,342 12/12 Internal Examiner for PhD qualifying exam, Internal Examiner for M.Sc. and PhD oral exam, Former Head, Department Chemistry OAU, Former Dean, faculty of Science OAU, Member, University Governing Council Member, University Development Committee Former Head, Chemistry Department, University of Ilorin Former Ag. Head, P/A Chemistry and SLT Departments LAUTECH. 10. Dr. (Mrs.) M.A. Oladipo Reader CONUASS 6 step 03 11/12/1995 F/T B.Sc. (Hons.) Chemistry. 1992, M.Sc. Inorganic Chemistry 1995, P.hD. Inorganic Chemistry 2002. CSN, STAN, ACS 23 Publications Teaching and Research: LAUTECH 1995 to date CHM 211, 311, 312, 558, 713 11. Dr. I.A. Bello Reader CONUASS 06 step 02 01/06/2004 F/T B.Sc. Industrial Chemistry 1983, M.Sc. Physical Chemistry 1988, Ph.D. Physical Chemistry 2004 CSN, STAN 19 Publications Teaching & Research: ABU Zaria 1991-2004 Teaching & Research: LAUTECH 2004 – to date. CHM 231, 331, 332, 512 12. Dr. O.S. Amuda Reader CONUASS 06 step 02 28/09/1998 F/T B.Tech. (Hons) Chemistry 1996, M.Sc. Analytical Chemistry 2001, Ph.D. Analytical/ Environmental Chemistry 2006., CSN, ACS, EDS 49 Publications Teaching & Research: LAUTECH 1989–to-date. Teaching & Research (sabbatical) 2008-2009 WUSTO Ondo. CHM 341, 342, 441, 463, 541, 561 46 8/8 12/12 12/12 Former Acting Head, Department of P/A Chemistry, Level Adviser, 1997 Set, Departmental Examination Officer, Chairperson: LAUTECH Guest House, Congregation representative to Senate, Member, Pre-degree Science Board, Faculty of Science Handbook Review Committee, Departmental Board of Examiners, Faculty Board of Examiners, Departmental Review Committee, Examination Moderator at Emmanuel Alayande College of Education Oyo. Former Acting Head, Level Advisor, 1999 Set Member, Publication Committee Examination Officer, 2006-2010, Deputy Dean, FPAS 2008-date Chairman, Departmental NUC Accreditation Committee Member,Departmental Publication Committee Research Project/ Seminar Coordinator Ag. Head Department of Chemical Sciences, WUSTO Ondo (sabbatical leave), Senate 13. Dr. (Mrs.) O.O.E. Onawumi Reader CONUASS 06 step 02 F/T 14. Dr. S.O. Oladoye Reader CONUASS 06 step 01 27/12/1995 F/T 15. Dr. (Mrs.) M.O. Bello Reader CONUASS 06 step 01 23/02/1999 F/T B.Sc. (Hons) Chemistry 1994, M.Sc. Analytical Chemistry 1997, Ph.D. Analytical/ Inorganic Chemistry 2006, CSN, PICCON, TWOWS 24 Publications B.Ed. (Hons) Chemistry 1990, M.Sc. Organic Chemistry 1993, Ph.D. Organic Chemistry 2006. CSN, STAN. 15 Publications B.Sc. (Ed.) Chemistry 1991, M.Sc. Industrial Chemistry 1995, Ph.D. Industrial Chemistry 2007 CSN, ICCON 20 Publications Rep. Appointment, and Promotion Committee, WUSTO, Ondo, Level Advisor, 2007 Set. VC Rep. Governing Council, OSCOTECH, Esa-Oke. Dean’s Rep, College of Heath Sciences Review Board. Member, Comm. On Review of University Handbook. External Examiner, Institute of Ecology and Environmental Studies, OAU, Ile-Ife. Level Advisor, 2008 Set Member, Examination committee, FPAS Member, Exhibition Committee, FPAS Treasurer, NAWACS Teaching: Walbrook College, Ibadan,1996-1997 Teaching : Unity Sec. Sch, Igboho 1998 (Jan-Nov) Teaching & Research: LAUTECH 1998 - date CHM 341, 342, 441, 558 12/12 Teaching: Alege Gram. Sch, Obudu, Kwara State 19901991 Teaching: A-Z Int. Sch. Lagos 1993-1995 Teaching: ADRAD Int. Sch. Lagos 1995 (Sept-Dec) Teaching & Research,: LAUTECH 1995 – to date. Teaching: St. Gregory College, Lagos 1992-1995, Teaching : Ogbomoso High Sch., Ogbomoso 1995-1996, Teaching : Anglican Gram. Sch. 1996-1999 Teaching & Research: LAUTECH 1999 - date CHM 222, 324, 321, 522 12/12 Chairman, University Car loan Committee Examination Officer 2004-2008 Level Advisor, 2001 Set Ag. Head P/A Chemistry Dept Aug 1- Aug 31, 2006. CHM 252, 351, 352, 354, 457, 401 12/12 Member, Postgraduate/Project Committee Member, University Time-Table & Examination committee Chairperson, Departmental Welfare Committee 47 SIWES Supervisor, 20052009 Level Advisor, 2007 Set 16 Dr. O.M. Olabemiwo Reader CONUASS 06 step 01 01/06/1995 F/T B.Sc. (Hons) Industrial Chemistry 1990, M.Sc. Industrial Chemistry 1995, Ph.D. Chemistry 2008, CSN 17 Publications Quality Control Officer: Shagoya Nig. Ltd., Lagos 1990-1991 Chemical Analyst Sam. Pharm. Ltd., Ilorin, 19911992 Teaching & Research: Fed. Poly. Ado-Ekiti 1993 - 1995 Teaching & Research: LAUTECH 1995 – date CHM 353, 355, 463, 252, 352, 354, 512, 642, 754 12/12 Member, Departmental Examination committee Member, University Admission Committee, FPAS Committee on Consultancy Services FPAS Committee on Postgraduate Studies 17. Dr. O.S. Bello Senior Lecturer CONUASS 05 step 02 23/02/1999 F/T Teaching & Research: LAUTECH 1999 - date CHM 231, 331, 433, 532, 612. 724 12/12 Level Adviser, 2004 Set Member, Departmental Examination Committee Member, LAUTECH 20th Anniversary Celebration Committee 18. Dr. T. A. Adedosu Senior Lecturer CONUASS 05 step 01 23/02/1999 F/T B.Tech. (Hons) Chemistry 1997, M.Sc. Physical Chemistry 2002, Ph.D. Physical Chemistry 2008, CSN, ACS 34 Publications B.Tech. (Hons) Chemistry 1997, M.Sc. Organic Chemistry 2001, Ph.D. Organic Geochemistry 2009, CSN, NAPE, OGAN 25 Publications Teaching & Research: LAUTECH 1999 - date CHM 222, 321, 421, 453, 523, 701, 721 12/12 19. Mrs. T.I. Edewor F/T CHM 222, 322, 324, 326, 453, 523 12/12 Dr. (Miss) F.A. Adekunle B.Sc. (Hons) Chemistry 1987, M.Sc. Chemistry 1997, Ph.D. (In View) 16 Publications B.Tech. (Hons) Chemistry 1997, M.Sc. Inorganic Chemistry 2002, Ph.D. Inorganic Teaching and Research: LAUTECH 1991 to date 20. Senior Lecturer CONUASS 05 step 02 06/11/1991 Senior Lecturer CONUASS 05 step 02 23/08/1999 Former Examination Officer, Level Adviser2005 Set, Member, TimeTable & Examination committee Member, LAUTECH 20th Anniversary Celebration Committee, Member, Departmental NUC Accreditation Committee, Member, Department Board of Examiners Member, Faculty Board Teaching & Research: LAUTECH 1999 - date CHM 211, 311, 312, 514 12/12 F/T 48 Level Adviser, 2003 Set Coordinator, Industrial attachment Member, Time-Table & 21. Dr. B. Semire Senior Lecturer CONUASS 05 step 01 30/01/2005 F/T 22. Mrs. A.O. Ibrahim Lecturer I CONUASS 04 step04 30/09/1998 FT 23. Mr. M. AbdulHammed Lecturer I CONUASS 04 Step 01 08/03/2005 F/T 24. Mr.A.A. Giwa Lecturer I CONUASS 04 step 01 10/05/2005 25. Mr. O.A. Popoola Assistant Lecturer CONUASS 2 Step 2 11/04/2006 Chemistry 2007, CSN 7 Publications B.Tech. (Hons) Chemistry 1999, M.Tech. Physical Chemistry 2004, Ph.D. Physical Chemistry 2008 6 Publications B.Tech. (Hons) Chemistry 1996, M.Sc. Analytical Chemistry 2001, Ph.D. (In View), CSN, ICCON 13 Publications Examination committee Teaching & Research: LAUTECH 2005 - date CHM 231, 431, 433 Teaching & Research: LAUTECH 1998 to date CHM 241, 341, 561 12/12 Member Departmental Registration Committee, Member, Departmental Welfare Committee, Member Departmental Project Committee, Member, Student Welfare Advisory Board B.Tech. (Hons) Chemistry 2003, M.Sc. Physical Chemistry 2006, Ph.D. (In View) CSN 10 Publications Teaching & Research: LAUTECH 2005-date Research Assistant UniBonn, Germany 2008-2009 (Study leave) CHM 231, 431, 457, 352 8/8 F/T B.Sc. (Hons) Industrial Chemistry 1987, M.Sc. Analytical Chemistry 2002, Ph.D. (In View) CSN, STAN 6 Publications Teaching & Research LAUTECH 2005 to Date CHM 401, 241,222 8/8 Level Adviser, 2009 Set, Departmental Time Table Officer, Member, Faculty Website development Committee, Secretary, Departmental NUC Accreditation Committee, MemberStudent Project Seminar Committee. Member, Research Project Committee, Member, Bus committee Member, Maintenance and Repair Committee F/T B.Tech. (Hons) Chemistry 2004, M.Sc. Physical Chemistry 2008, Ph.D. (In View) CSN, ACS, Teaching & Research: LAUTECH 2006-date On Study Leave On Study Leave 49 10/10 Adviser to SCSN Level Adviser, 2003 Set Member, Departmental Examination Committee, Member-Student Project Seminar Committee. On Study Leave at Cambridge University, 26. 27. Mr. A.J. Adepoju Mr. A.O. Esan Assistant Lecturer CONUASS 02 step 01 29/12/2009 F/T Assistant Lecturer CONUASS 02 step 01 29/12/2009 F/T CamBridgeSens 2 Publications B.Tech. (Hons) Chemistry 2003, M.Tech. Organic Chemistry (In View) CSN B.Tech. (Hons) Chemistry 2007, M.Sc. Industrial Chemistry (In View) CSN U.K. Teaching: Smith Int. Baptist Academy, Ogbomoso 20052009 Research and Instruction during Practical Classes: LAUTECH 2009 to Date Research and Instruction during Practical Classes: LAUTECH 2009 to Date 50 CHM 191, 192, 291, 292 4/4 Member-Student Project Seminar Committee. CHM 191, 192, 291, 292 4/4 Member-Student Project Seminar Committee. 51. Table 8: Laboratory Staff Complete the table below in respect of laboratory staff available for the various laboratories used for teaching the Programme/Sub- discipline/Discipline. S/NO. Name 1. Mr. O. Z. Olawuyi 2. Mr. O. K. Fakorede 3. Mr. K.A. Adebisi 4. Mr. P.A. Omolere 5. Mr. J.A. Olajide Rank/Designation Date of First Appointment Assistant Chief Technologist CONTISS 12 step 04 14 /12/ 1995 Qualifications, Dates Obtained, Membership of Professional Association Final Diploma 1993, HND (NIST) ANIST, PGD Food Chemistry 2004. Principal Technologist CONTISS 11 step 03 12 /04/2001 Technologist I CONTISS 08 step 05 01/10/1990 Technologist II CONTISS 07 step 03 03/09/1990 HND 1988, PGD Food Chemistry 2003 ANIST Senior Lab. Supervisor CONTISS 06 step 29/10/1990 MOD III Certificate 1966 CERT in Bookshop Practices 1974 ND 2002, HND 2004 ND 2003, HND (NIST) 2005 51 Duties Performed/Courses Taught He is in charge of 100 level laboratory practical and is saddled with the responsibilities of maintenance and repair of equipments used for the practical. He supervises the junior technical staff in the department and trains students on SIWES programme. He is also involved in taking inventory of scientific equipments in laboratories and also assist the lecturers and students in research project work. He is a member of Departmental Review Panel and Finance Committee. Member, Departmental NUC Accreditation Committee In charge of 200 level practical, and in the maintenances and repair of equipment used for the practicals. He trains and supervises the junior technical staff under him and students of the department and other institutes. He is a member of Departmental Maintenance Committee. He is in charge of 100 level practical, he prepares the reagents for the level He handles the equipments in the lab and also assists the final year students in their project works. In charge of the preparation of reagents for 200, 300 and 400 Level students’ practicals. He maintains the equipments in the lab and handles them perfectly. He assists in research projects of the lecturers and students in their final year. He is a member of Social Committee of the department. He assists in the preparation of reagents and materials for 100 level practical classes. He supervises the cleaning of the laboratory apparatus and setting up of the lab for the practicals. He keeps the records of manuals and practical notebooks of students. 6. Mr. A.B. Olawore 7. Miss H.I. Bello 8. Mrs. I.A. Olaiya 9. Mr. S.B. Akanji 10. Miss O. Akinboade 11. Mrs. A.N. Onyiaoha 12. Mrs. F.T. Ajayi Laboratory Assistant Technologist CONTISS 06 step 03 27/01/1993 Laboratory Supervisor CONTISS 05 step 06 14/11/1995 Laboratory Supervisor CONTISS 05 step 06 22/11/1995 Laboratory Supervisor CONTISS 05 step 05 27/11/1995 Snr. Lab Assistant CONTISS 04 step 05 19/10/1998 Laboratory Assistant CONTISS 03 step 06 15/06/2000 Lab Assistant CONTISS 03 step 04 02/05/2001 ND 2002, HND (NIST) 2007 He assists i n the preparation of reagents and materials for 100 and 200 levels practical classes. He is also involved in the maintenance of lab apparatus and supervises 100 and 200 level students in their practicals. WASC 2000, ND 2005 She has been assisting in the preparation of laboratory reagents for 100 level practical classes and helps in conducting such. She keeps the records of manuals and practical notebooks of students. She also helps the final year students in their project works. She is a member of Social Committee. She has been assisting in the preparation of laboratory reagents for the practical classes of students 100 level. She keeps the records of students and maintains the glass wares. She also sets up the apparatus and equipments for practical and asists the final year students in their project works. He is involved in the preparation of reagents and conducts practical for 200, 300 and 400 level students’ practical. He assists the students in their project works and assists in keeping the store of the department. He also repairs the electrical appliances in the department. She assists in the preparation of laboratory reagents and in conducting practical classes of students in 200, 300 and 400 level. She maintains and cleans the laboratory glass wares and benches. NECO 2003, ND 2005 ND 2003, HND 2005 NECO GCE B. Edu (Tech/ Env education) 2005 Word Processing and Desktop Publishing 2006 She is involved in the conduct and preparation of reagents of 100 level student practical. She assists the final year students in their project works. She cleans and maintains the laboratory glass wares, benches and equipments. NECO 2002, ND 2004, HND 2006 She assists in the conduct of 100 level practical classes. She also assists in cleaning and maintaining the laboratory apparatus and benches. She keeps laboratory manuals and notebooks of students. She assists the technologists in reagent preparation. 52 52. Table 9: Administrative Non-teaching Staff Disposition in the College/School/Faculty/Department where programme/ Sub-discipline/Discipline to be Accredited is offered. Supply the information in the table below: Use additional sheets, if necessary. S/NO. Name 53. 1. Mrs. Ojo Olamide 2. Mrs. F. E. Oluwasina 3. Mr. A.O. Ige 4 Mr. S. Abobade Rank/Designation Salary Scale and Date of First Appointment Qualification and Dates Obtained Post Qualification Work Experience Remarks Conf. Sec. CONTISS 07 step 4 01/10/1995 Data Management Officer. CONTISS 08 step 5 Clerical Officer CONTISS 07 step 03 24/06/1994 HND (Secretarial Admin.) Certificate in Words Processing and Desktop Publishing 2003. NCE (BNS. Edu.) B.Ed (G&C), Certificate in Sec. Studies, Cert. in WP/DP) Conf. Sec. I LAUTECH 2006 to date. Satisfactory DMO I Satisfactory OND Public Admin 2001 Clerical Officer LAUTECH: 2005 to date Satisfactory Senior O. A. WAEC WAEC Satisfactory Staff Appraisal: Appraise the entire academic staff of the programme/sub-discipline/discipline. (a) adequacy in number, qualification and experience (State average student to staff ratio) The strength of the academic staff has increased to the level that can meet the demand of students’ population. Nineteen academic staff out of twenty-seven have Ph.D., five are about to complete their Ph.D. programme. All the Lecturers are qualified to teach in the University; they are experienced. The average students/staff ratio is 17:1. 53 (b) Effectiveness of Lectures The staffs are quite effective as Lecturer. Their attendance at Lectures, Laboratory, practical, tutorials and seminars is high and satisfactory. They give and grade tests and assignments to students as demanded by the curriculum. (c) Professional Achievements As Chemists, the staffs have participated actively in the seminars and conferences of notable societies such as Chemical Society of Nigeria (CSN). Some are members of Institute of Chartered Chemists of Nigeria (ICCON), Nigerian Association of Petroleum Explorationist (NAPE), New York Academy of Sciences (NYAS), European Desalination Society and Shell International Global Network on Climate Change (SIGNCC) among others International Associations and Learned Societies. They have contributed to the Chemistry profession by way of publications in Local and International Journals; by serving as External Assessors for promotion, External Examiners to other Universities, Colleges of Education and Polytechnics. 54 54. Table 10. Facilities Available to the Department offering Programme to be accredited Complete the Table shown below: Type of Facility No. Available Average area of Room/studio etc. in m sq. No. of students each room can accommodate No. of rooms jointly used with other Departments Expansion Programme (if any) Additional facility A B C D Year Started Year of Completion G H F Total Facility that will be available to Department when expansion work is completed (B + F) E 1. Lecture Room 3 2. Lecture Theatre 5 3. Assembly/Exams Hall - Ranges from 100m.sq To 200m.sq Ranges from 200m.sq 5000m.sq - 4. Laboratories Old Chemistry Lab. 2 3500m sq. Old new Chemistry Lab. 1 2500m sq. New Chemistry Lab. 2 5. Workshops 2 200m.sq Ranges from 250 - 1000 3 1 2008 - Ranges from 100 - 1200 5 1 2008 - 4 6 New Chemistry Laboratory Complex to accommodate 800 students and office accommodation for 24 staff. 100 -400 100 1 55 Under the New laboratory Complex, there will be 5 July 2007 March 2008 July 2007 March 2008 workshop room with adequate facilities 6. Studios 7. Library 1 8. Office Accommodation 18 5,000m.sq 16.5m.sq 2,500 1 – 2 staff Five additional room offices annex and New Chemistry Laboratory Complex will contain 12 office rooms 1Prof - 1 Office 1Raeder – 1” 1SL - 1 Office 1L1 - 1 Office Other academic staff shared office 9. Office Accommodation 10. Other (Specify) 1 - 200m.sq Five additional room offices annex and New Chemistry Laboratory Complex will contain 12 office rooms 1 – 2 staff - 56 Nov. 2006 March 2007 July 2007 March 2008 May, 2009 Oct., 2009 36 40 55. Laboratories Describe the various laboratories available for teaching the programme. Indicate if the laboratories belong to the Department or shared with other Departments. List the equipment in each laboratory using the table in APPENDIX 1 of this Form. S/No 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. SOLID REAGENTS Acetanilide Ammonium oxalate DL camphor Diphenylamine LR D-(+)-Gluscose Lead (II) acetate 1-Naphthol-4-sulphonic acid 2-Naphthol 1-Naphthol Pepsin powder Naphthalene Oxalic acid Potassium sodium (+) tartrate powder Potassium sodium tartrate Picric acid Tartaric acid Thiourea Sodium ethanedioate Sodium (+) tartrate Soluble starch Sodium acetate Zinc sulphate Potassium cyanide Potassium ferricyanide Calcium nitrate Cupric chloride Potassium chromate Sodium chloride Sodium bromide Sodium dichromate Sodium tetraborate powder Magnesium nitrate Manganese (II) chloride Calcium carbonate Barium hydroxide Barium Nitrate Barium sulphate 57 QUANTITY 4x500g 1x500g 2x500g 1x250g 1x250g 1x250g 1x100g 6x100g 3x500g 1x250 2x500g 4x500g 1x500g 1x500g 1x100g 1x500g 1x500g 1x500g 1x500g 1x500g 2x500g 2x1000g 1x500g 2x500g 3 x 500g 3 x 500g 2 x 500g 2 x 500g 1 x 500g 2 x 500g 3 x 500g 1 x 500g 1 x 500g 2 x 500g 1 x 500g 2 x 500g 1 x 500g 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. Boric acid Disodium tetraborate Potassium diphosphate Lead acetate Lead chloride Iron metal Potassium dichromate Potassium chloride Potassium iodide Potassium chromate Ammonium iron (II) sulphate Ammonium thiocyanate Ammonium carbonate Alumininium potassium sulphate Aluminium oxide (neutral type E) Potassium phosphate Potassium iodate Potassium hydrogen carbonate Silica gel white (coarse) Sodium carbonate hydrated Ferrous sulphate crystalline Calcium carbonate Ammonium oxalate Potassium sulphate Zinc chloride Sodium nitroprusside Phenol crystal 2 x 500g 1x500g 1 x 500g 1 x 500g 1 x 500g 1 x 500g 3 x 500g 1 x 500g 1 x 500g 2 x 500g 2 x 500g 1 x 500g 1 x 500g 1 x 500g 6 x 500g 5 x 500g 2 x 500g 5 x 500g 7 x 500g 4 x 500g 6 x 500g 4 x 500g 4 x 500g 5 x 500g 1 x 500g 1 x 500g 1 x 500g S/No 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. LIQUID REAGENTS Parrafin Liquid Methylated spirit Acetone Diethyl ether Acetic anhydride n-hexane Sulphuric acid Hydrogen peroxide Ammonia solution Hydrochloric acid Glacial Acetic acid Methanol Nitric acid Triethanol amine QUANTITY 1 x 2.5 liters 6 x 2.5 liters 1 x 2.5 liters 10 x 2.5 liters 7 x 2.5 liters 2 x 2.5 liters 1 x 2.5 liters 1 x 2.5 liters 1 x 2.5 liters 1 x 2.5 liters 3 x 2.5 liters 2 x 2.5 liters 1 x 2.5 liters 1 x 500 ml 58 APPARATUS TEST TUBE BURETTES PIPETTES BEAKER ROUND BOTTOM FLASK FLAT BOTTOM FLASK CONICAL FLASK MEASURING CYLINDER REAGENT BOTTLE FUNNEL INDICATOR B0TTLE BUCKNER FUNNEL REFLUX APPARATUS VOLUMETRIC FLASK ORGANIC SET QUICKFIT COLUMN CAPACITY 1620/22m 1620/09m 1620/10m 1620/02m 50 mls 10 mls 25 mls 100 mls 250 mls 600 mls 400 mls 10 mls 50 mls 2000 mls 500 mls 100 mls 250 mls 1000 mls 1000 mls 500 mls 100 mls 250 mls 500 mls 1OOO mls 2000 mls 10mls 20 mls 500mls 250 mls 50 mls 150 2000 500 50 mls 127C/1 127C/2 1000 mls 500 mls 250 mls Semi Micro 22 59 QUANTITY 1100 1600 2900 100 6 75 75 90 24 268 17 50 25 14 36 96 120 30 10 30 48 62 10 12 6 25 55 45 240 72 12 6 72 77 20 14 4 20 48 72 2 THERMOMETER 0 -110 °C 0 – 250 °C 0 – 360 °C 70 10 20 EQUIPMENT HOT PLATE & STIRER HOT PLATE CENTRIFUGE SENSITIVITY BALANCE WATER BATH STIRRER pH METER TOTAL NO 2 3 8 1 3 1 2 NOTE: ALL THE EQIUPMENT/APPARATUS LISTED ARE IN GOOD CONDITION 60 56. Clinics/Studios Describe the types of clinics/studios, if any, available for the programme, indication if they are specific to the Department or shared with other Departments. List the equipment etc in each clinic/studio using the table in APPENDIX 1 of this Form. NOT APPLICABLE 61 57. Office Accommodation for Staff Comment on the office accommodation situation for academic staff, stating the size of accommodation, list of furniture items and how many lecturers share the rooms. Office Accommodations are available at the Department main Office and two Laboratories (Old and New Chemistry Labs). There are Twenty-three office rooms each of average size 5m X 4m. From the rank of Senior Lecturers up to Professors, Lecturers are alone in their offices. Assistant Lecturers/Graduate Assistants share office rooms, maximum of two staff per office. A typical office has the following furniture: 1. 1No. writing table with drawers 2. 1 No. Armchair 3. 2 No. Visitor’s chairs 4. 1 Easy chair 5. 1 Book shelve 6. 1 Notice board 7. Ceiling fan 8. Air-conditioner system 9. White marker board 62 58. Appraisal of Facilities Appraise the existing facilities in term of quality and quantity for current and projected enrolment period. For the present enrolment, the available space and equipment are adequate for teaching and research. However, the construction of more laboratories and Lecture spaces is underway, by the University Management. There is also plan to acquire more modern equipment for the undergraduate practical in line with the NUC guideline. 63 59. Table 11: College/School/Faculty/Department Finances: Recurrent Income and Expenditure Complete the table shown below for the college/School/Faculty/Department in three years preceding the one in which the programme to be accredited offered. Academic Year Year 2006/2007 2006/2007 2007/2008 2008/2009 Amount in N Amount in N Amount in N Cost Category Year 2007/2008 Year 2008/2009 Provision Actual Expenditure Provision Actual Expenditure Provision Actual Expenditure N N N N N N 1. Staff Salaries 34,381,278.48 34,381,278.48 37,556,924.93 36,216,378.6 41,601,813.62 41,601,813.62 2. Staff development 1,310,508.61 1,310,508.61 1,560,374.47 1,560,374.47 1,887,578.04 1,887,578.04 Sources of funds University’s Budgetary Allocation to the College/School/ Faculty/ Department 35,691,787.09 37,776,753.07 Consultancy Voluntary Public support Seminar Fees CENTRALISED Tuition Fees where Applicable Others (Specify) Total 43,489,391.66 3. Library Materials 4. Laboratory Equipment 5. Studio Equipment 6.Office/Class Furniture 7.Maintenance 8. Supplies/ Training Consumables 9. Vehicles Maintenance 10. Utility Services 11. Research 12. Other (Specify) Total CENTRALISED 64 60. Table 12: Capital Funds: Provision and Expenditure Complete the Table shown below for the four years preceding the one in which the accreditation is being undertaken Year 2005/2006 Year 2006/2007 Year 2007/2008 Year 2008/2009 Provision Provision Provision Provision Expenditure Expenditure Expenditure Expenditure 1. Expansion to Physical Facilities Category a. Classroom Lecture Theatre b. Laboratory/ Workshop Studio Capital Development is handled centrally from the Physical Planning Unit of the Vice-Chancellor’s Office; Funds are not Allocated to the Department directly. 2. Machines and Equipment 3. Others (Specify) 65 61. Assets and Liabilities State below the current Assets and Liabilities of the College/School/Faculty/Department. The assets of the Department are in form of buildings, electricity generating plants and equipment. There are no liabilities. 62. Financial Appraisal Appraise the adequacy of the operating Funds for the College/School/Faculty/Department. For recurrent expenditure also indicate the expenditure per annum per student. The Staff salaries are paid as and when due. The department is run on quarterly imprest of N 105,000:00 available from Bursary department of the University. The money is used for photocopy of official documents and entertainment at Departmental meetings. The University Management and Postgraduate School sponsored staff for Local and International Conferences. However, Chemistry department depends on the Departmental internally generated revenues of about 1.5million per annum to procure some office needs (i.e. Computer Systems, Toner Cartridges, UPS, Stabilizers, photocopy of official documents, Extension of Office space, repairs and maintenance of the departmental properties etc). The faculty also procures office equipment for departmental examination processing. 63. Appraisal of Standard of Degree Examination Appraise the standard of examination based on: (a) adequacy of coverage of the syllabus content All students take the compulsory courses plus enough elective and required courses to make up the minimum credits for the award of a degree in Pure and Applied Chemistry. The syllabus in each course is properly covered and examination questions are set to cover the range of topics in the syllabus. (b) quality of students’ answers to the various questions The students have performed well in their examinations and the department has been able to graduate 4 first class and several second class upper students in its 16 years of existence. (c) quality of practical work, continuous assessment and degree projects The quality of the practical work is high and the degree projects have been well commended by the external examiners. 66 (d) students’ readiness for the level of manpower he/she is being trained for Graduates from the department have fared well in their careers. Those that have gone on to post graduate have also done very well both within and outside the country. (e) external moderation scheme. External Examiners have been satisfied with the standard of examination and the quality of the candidates presented to them. 67 64. Table 13: Employer’s rating of Graduates of Programme/Sub-Discipline to be accredited Complete the Table below for 24 graduates of Programme/Sub-discipline to be accredited for each of the three years preceding the Accreditation visit. S/No. Year of Graduation Name of Graduates Programme 1 2 3 1. 1996/1997 AMUDA, Sarafadeen Omotayo B. Tech 2. 1996/1997 OLAWALE, Asiata Omotayo B.Tech 3. 1997/1998 ADEDOSU, Taofik Adewale B. Tech 4. 1997/1998 BELLO, Solomon Olugbenga B. Tech 5. 1997/1998 ADEKUNLE, Funmilayo Abosede B.Tech 6. 1998/1999 SEMIRE, Banjo B.Tech 7. 2001/2002 ABDUL-HAMMED, Misbaudeen B.Tech Name of Employers or Universities attended by Graduates 4 Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso 68 Appointment Summary of Employers’ Remarks 6 5 Reader Satisfactory Lecturer I Satisfactory Senior Lecturer Satisfactory Senior Lecturer Satisfactory Senior Lecturer Satisfactory Senior Lecturer Satisfactory Lecturer I Satisfactory 8. 2002/2003 POPOOLA, Olalekan Abdulmuiz B.Tech Assistant Lecturer Satisfactory Graduate Assistant Satisfactory Graduate Assistant Satisfactory B. Tech Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso Department of P/A Chemistry, LAUTECH, Ogbomoso CAPL Nig. Ltd, Lagos 9. 2001/2002 ADEPOJU, Adewusi John B.Tech 10. 2006/2007 ESAN, Akintomiwa Olumide B. Tech 11. 1998/1999 ORONIRAN, Oluiwakemi Marketing Manager Satisfactory 12. 1998/1999 ADETOMIWA, Yemi B.Tech CAPL Nig. Ltd, Lagos Satisfactory 1998/1999 AJALA, Abayomi B. Tech SON, Oshogbo Branch Production Manager Research Officer 13. 14. 2005/2006 AFOLAYAN, Omolola B. Tech NAFDAC, Lagos Branch Research Analyst Satisfactory 15. 1998/1999 OSIKAYODE, Akinlawon B.Tech ETB, Bayelsa State Satisfactory 16. 2001/2002 ADEYEMO, Omowumi F. B.Tech PZ Nig. Ltd., Lagos 17. 2001/2002 ALABI, Oyedokun B.Tech Sweetco Nig. Ltd, Ibadan 18. 1999/2000 YUSUFF, Kamil Olaniyi B.Tech 19. 2001/2002 OYEBISI, Rotimi O. B.Tech 2001/2002 SANNI, Akeem A. B. Tech Customer Care Service Lecturer Satisfactory 20. 21. 1998/1999 OLOKO, Ayomide B.Tech Lagos State University, LASU, Lagos GlobaCom Nig. Ltd. Lagos Kogi State College of Education, Kogi State Tai Solarin University Of Education, Ijebu-Ode Assistant Branch Manager Assistant Marketing Manager Quality Control Manager Assistant Lecturer Graduate Assistant Satisfactory 69 Satisfactory Satisfactory Satisfactory Satisfactory Satisfactory 22. 2001/2002 ZACCHAEUS, Sunday A. B.Tech Bells University, Ota Assistant Lecturer Satisfactory 23. 2001/2002 OLAITAN, Azeezat O. B. Tech Chemistry Teacher Satisfactory 24. 1999/2000 OPATOKUN Suraj A. B.Tech Islamic College, Ogbomoso University Sains Malaysia Ph.D. Student Satisfactory 70 65. Table 14: List of Principal Tools, Machines, Instruments and Equipment Available Complete the table below in respect of the above Use separable sheets with the same headings if necessary Name of Laboratory/Clinic/Studio ……………………………………………….. Item No. Description of items Quality in Stock (Usable items only) Remarks THIS INFORMATION HAS BEEN PROVIDED UNDER SECTION 55 Total cost of usable items available at the time of completing questionnaires 71 NAME OF OFFICER COMPLETING THE FORM: Name: Dr. I. O. Adeoye Rank: Reader/ Ag. Head of Department Signature ………………………………. Date: …………………………… Name: Prof. E. T. Ayodele Rank: Professor/ Dean, Faculty of Pure &Applied Sciences Signature ………………………………. Date: …………………………… 72