Rituxan for RA CC
advertisement

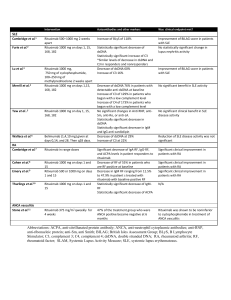
Utilization Review Policy Subject: rituximab injection for IV use (Rituxan®) in Rheumatoid Arthritis Date revised: 12/02/2009 Description Rituximab is a monoclonal antibody directed against the CD20 antigen.1 Rituximab in combination with methotrexate is approved by the Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis (RA). Rituximab is also FDA-approved for the treatment of non-Hodgkin’s lymphoma and is used for many off-label indications. This policy addresses its use in RA. Rituximab is available as a preservative-free liquid concentrate for intravenous (IV) administration (infusion, not IV push). It is available in a concentration of 10 mg/mL in either 100 mg (10 mL) or 500 mg (50 mL) single-use vials. The dose is diluted before IV infusion. The rate of the infusion is gradually increased as tolerated up to a maximum rate of 400 mg/hour. If an infusion reaction occurs, the infusion is interrupted or the rate is decreased. If necessary medical management of the reaction is used. In RA patients, premedication with methylprednisolone 100 mg IV or an equivalent glucocorticoid is recommended 30 minutes before the rituximab infusion to reduce the incidence and severity of infusion reactions. Patients are also pre-medicated with acetaminophen and an antihistamine. Indications, Medically Necessary 1. RA in adults: Indicated for the treatment of moderate to severe active RA in patients who meet all of the following criteria. Rituximab is prescribed by a rheumatologist, and Patient has had an inadequate response after at least 2 months of therapy with one of the following non-biologic DMARDs: methotrexate, hydroxychloroquine, leflunomide ® (Arava ), or sulfasalazine, and Exceptions to having tried a non-biologic DMARD for 2 months can be made if the patient could not tolerate the non-biologic DMARDs or if there are contraindications to all four of these agents. Exceptions to trying one of the non-biologic DMARDs listed above for 2 months can be made if all of the following conditions are met. The patient has recent onset RA within the previous 6 months AND meets one of the exceptions to trying a TNF antagonist (See the next criteria for trying a TNF antagonist for at least 2 months.) AND the patient has high disease activity as determined by the rheumatologist (document physician’s method of determining disease activity) OR the patient has a poor prognosis AND the patient will be on methotrexate in combination with rituximab unless there is a contraindication to methotrexate. Markers of poor 12/02/2009 1 Rituximab (Rituxan) prognosis include functional limitations based on the Health Assessment Questionnaire (HAQ) or other functional status measures; extra-articular manifestations of the disease (e.g., rheumatoid nodules, RA vasculitis, secondary Sjögren’s syndrome, Felty’s syndrome, RA lung disease); positive rheumatoid factor (RF) and/or positive anticitrullinated peptide (CCP) antibodies; and/or bone/joint erosions by radiography. Patients with early RA and low or moderate disease activity are usually not started on rituximab or another biologic DMARD (such as etanercept, adalimumab) as the initial therapy. Patient has tried a TNF antagonist, adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), golimumab (Simponi) or infliximab (Remicade) and had an inadequate response after at least 2 months of therapy or was intolerant to one of these TNF antagonists, and Exceptions to trying a TNF antagonist can be made in the following circumstances: Patients who have a contraindication to a TNF antagonist, especially those with a history of B cell lymphoma2 or Patients with a demyelinating disorder such as multiple sclerosis2 or Patients with a history of recurrent infections or Patients with a history of cancer. Rituximab will be used in combination with methotrexate. Rituximab is usually given in combination with methotrexate. Exceptions to using rituximab in combination with methotrexate can be made in the following circumstances: The patient has tried methotrexate and is intolerant or has a contraindication to methotrexate. Rituximab has been used in combination with leflunomide and as monotherapy for RA.2-4 For patients who are continuing with rituximab there must be documentation that the patient is or is not on another DMARD in conjunction with rituximab and the other drug therapies that have been used to treat RA. Dosing in RA: Two-1000 mg IV infusions separated by 2 weeks. Rituximab is given in combination with methotrexate. Safety and efficacy of retreatment have not been established in controlled trials. According to the product information, a limited number of patients have received two to five courses (two infusions per course) of treatment in an uncontrolled setting. In clinical trials in patients with RA, most of the patients who received additional courses did so 24 weeks after the previous course and none were retreated sooner than 16 weeks. Initial approval/extended approval: Initial approval is for the first 2 infusions of 1000 mg each. Six months or greater after the first dose of the previous rituximab regimen, approve another 2 doses if the patient has responded (e.g., less joint pain, morning stiffness, or fatigue; improved function or activities of daily living; decreased soft tissue swelling in joints 12/02/2009 2 Rituximab (Rituxan) or tendon sheaths, improved laboratory values; reduced dosage of corticosteroids), as determined by the prescribing rheumatologist. Rarely, some patients may have a response and then have a flare after 16 weeks. Exceptions can be made for these patients to have another 2 doses after 16 weeks and before 6 months. Limited information is available on patients who do not respond to the first course of rituximab. Patients who are responding to therapy with rituximab may receive additional courses of therapy with at least 6 months between courses. Duration of therapy in RA: indefinite in patients who are responding. Labs/Diagnostics required: none. Waste management: The initial dose in RA is two 1000 mg (2 of the 500 mg vials per dose) infusions separated by 2 weeks. Exclusions: Concomitant use with anakinra (Kineret), a TNF antagonist (such as etanercept, adalimumab, golimumab, certolizumab pegol, infliximab), abatacept (Orencia®), or natalizumab (Tysabri®). Significant active infection. Individuals of childbearing potential should use effective contraception during treatment and for 12 months after rituximab therapy. Severe heart failure (class IV).2 Juvenile idiopathic arthritis or juvenile rheumatoid arthritis. Ankylosing spondylitis. Psoriatic arthritis. REFERENCES 1. 2. 3. 4. Rituxan injection [package insert]. South San Francisco, CA: Genentech, Inc; October 2009. Blank N, Max R, Schiller M, et al. Safety of combination therapy with rituximab and etanercept for patients with rheumatoid arthritis. Rheumatology. 2009;48:440-441. Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572-2581. Owczarczyk K, Hellmann M, Fliedner G, et al. Clinical outcome and B cell depletion in patients with rheumatoid arthritis receiving rituximab monotherapy in comparison with patients receiving concomitant methotrexate. Ann Rheum Dis. 2008;67:1648-1649. Cohen SB, Emery P, Greenwald MW, et al; REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to antitumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216-221. Finckh A, Ciurea A, Brulhart L, et al. Which subgroup of rheumatoid arthritis patients benefits from switching to rituximab versus alternative anti-TNF agents after previous failure to anti-TNF agent. Ann Rheum Dis. 2010;69:387-393. Rubbert-Roth A and Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11(Suppl1):S1-S12. Strand V, Balbir-Gurman A, Pavelka K, et al. Sustained benefit in rheumatoid arthritis following one course of rituximab: improvements in physical function over 2 years. Rheumatology (Oxford). 2006;45:1505-1513. Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al; DANCER Study Group. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390-1400. 12/02/2009 3 Rituximab (Rituxan) Mease PJ, Revicki DA, Szechinski J, et al. Improved health-related quality of life for patients with active rheumatoid arthritis receiving rituximab: Results of the Dose-Ranging Assessment: International Clinical Evaluation of Rituximab in Rheumatoid Arthritis (DANCER) Trial. J Rheumatol. 2008;35:20-30. Tak PP, Rigby W, Rubbert A, et al. Inhibition of joint damage and improved clinical outcomes with a combination of rituximab (RTX) and methotrexate (MTX) in patients with early active rheumatoid arthritis (RA) who are naïve to MTX: a randomized active comparator placebo-controlled trial. Ann Rheum Dis. 2009;68(Suppl 3):75 [abstract OP-0022]. Rigby WF, Ferraccioli G, Greenwald M, et al. Rituximab improved physical function and quality of life in patients with early rheumatoid arthritis: results from a randomized active comparator placebo-controlled trial of rituximab in combination with methotrexate compared to methotrexate alone. Ann Rheum Dis. 2009;68(Suppl 3):581 [abstract SAT0121]. Teng YKO, Tekstra J, Breedveld FC, et al. Rituximab fixed retreatment versus on-demand retreatment in refractory rheumatoid arthritis: comparison of two B cell depleting treatment strategies. Ann Rheum Dis. 2009;68:1075-1077. Higashida J, Wun T, Schmidt S, et al. Safety and efficacy of rituximab in patients with rheumatoid arthritis refractory to disease modifying antirheumatic drugs and anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2005;32:2109-2115. Keystone E, Fleischmann R, Emery P, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007;56:3896-3908. Popa C, Leandro MJ, Cambridge G, et al. Repeated B lymphocyte depletion with rituximab in rheumatoid arthritis over 7 yrs. Rheumatology (Oxford). 2007;46:626-630. Smolen JS, Keystone EC, Emery P, et al; Working Group on the Rituximab Consensus Statement. Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:143-150. Thurlings RM, Vos K, Gerlag DM, Tak PP. Disease activity-guided rituximab therapy in rheumatoid arthritis: the effects of re-treatment in initial nonresponders versus initial responders. Arthritis Rheum. 2008;58:3657-3664. Vital EM, Dass S, Buch MH, et al. How to manage non-response to rituximab: predictors and outcome of retreatment provide data for a treatment algorithm. Ann Rheum Dis. 2009;68(Suppl 3):77 [abstract OP-0027]. Genovese MC, Breedveld FC, Emery P, et al. Safety of biologic therapies following rituximab treatment in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:1894-1897. Furst DE, Keystone EC, Kirkham B, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2008. Ann Rheum Dis. 2008;67 Suppl 3:iii2-25. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762-784. Available at: http://www.rheumatology.org/publications/guidelines/recommendations.asp?aud=mem Accessed 11/16/2009. American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis. Arthritis Rheum. 2002;46:328-346. Available at http://www.rheumatology.org/publications/guidelines/raguidelines02.asp?aud=mem. Accessed on 11/16/2009. Abbreviations CCP = citrullinated peptide DMARD = disease modifying antirheumatic drug FDA = Food and Drug Administration HAQ = Health Assessment Questionnaire IV = intravenous mL = milliliter RA = rheumatoid arthritis RF = rheumatoid factor TNF = tumor necrosis factor Disease Modifying Antirheumatic Drugs (DMARDs). Generic Name Trade Name Traditional (Synthetic) DMARDs azathioprine (oral) generics, Imuran cyclosporine (oral) generics, Neoral, Sandimmune d-penicillamine (oral) Cuprimine, Depen gold compounds gold sodium thiomalate (injection) Myochrysine® auranofin (oral) Ridaura 12/02/2009 4 Rituximab (Rituxan) hydroxychloroquine (oral) leflunomide (oral) methotrexate [MTX] (oral, injection) minocycline (oral) sulfasalazine (oral) Biologic DMARDs abatacept (injection) adalimumab (injection) anakinra (injection) certolizumab pegol (injection) etanercept (injection) golimumab (injection) infliximab (injection) rituximab (injection) tocilizumab (injection) generics, Plaquenil generics, Arava generics, Rheumatrex generics, Minocin® generics, Azulfidine En-tabs, Azulfidine Orencia Humira Kineret Cimzia® Enbrel Simponi® Remicade Rituxan® Actemra® 12/02/2009 5