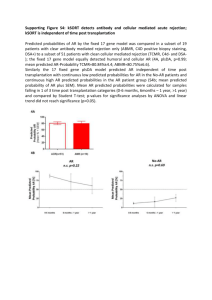
FEC_1917_sm_Captions-References
advertisement

Functional Ecology SUPPLEMENTARY FIGURE CAPTIONS Figure S1. Observed and predicted relationship between body size (snout vent length, SVL) and wet mass. The observed relationship comes from Fig. 2 in Tinkle and Ballinger (1972), mass = 3.793*10-5SVL2.983, R2 = 0.99. This implies a shape correction factor, δM, of 0.2474 (Table 1b). Figure S2. Predicted and observed (Tinkle & Dunham 1986) relationship between body size (measured as snout-vent length, SVL) at maturity vs. mean maximum body size. The predicted relationship sometimes exceeds the observed value, potentially because at some sites individuals do not survive long enough to reach maximum size. Figure S3. Example hourly output for the middle day of each month from the coupled biophysical/Dynamic Energy Budget models. Figure S4. Fits of observed (points) vs. predicted (lines) development time (a), assimilation rate (b) and resting metabolic rate (c) based on a common Arrhenius relationship (see Table S1 for parameters). Dotted lines show uncorrected Arrhenius responses (akin to Q10 responses) while solid lines show the Sharp adjustment (see Kooijman 2010 p. 21). Observed data are from (Hughes et al. 1982; Andrews et al. 2000; Angilletta 2001a, b). Figure S5. Predicted and observed resting oxygen consumption and assimilation rates. Part a) shows oxygen consumption rate plotted against wet body mass for a body temperature of 30 °C. The data points come from the literature for S. undulatus (Angilletta 2001a, b) and the related S. Metabolic theory, life history, and the distribution of a terrestrial ectotherm Michael Kearney Functional Ecology occidentalis (Dawson & Bartholomew 1956). The solid black line shows the DEB prediction and the light grey line shows the empirically estimated interspecific allometric curve for lizards (Andrews & Pough 1985). The thick black line shows a power function fitted to the DEB curve, which has a mass exponent of 0.82. The DEB prediction comes directly from the stoichiometric elements of the theory, assuming uric acid as the nitrogenous waste and that the molecular composition of food, structure, reserve and products have, for every carbon atom, 1.8 hydrogen atoms, 0.5 oxygen atoms and 0.15 nitrogen atoms (see section 4.31 of Kooijman 2010). Part b) shows the predicted vs. ‘observed’ assimilation rate, the latter coming either from Angilletta (2001a) (closed circles) or the polynomial function fitted by Waldschmidt and reported in Grant and Porter (1992) (open circles). The fine dotted line shows the 1:1 relationship. Figure S6. a) Predicted (lines) and observed (symbols) growth trajectories for laboratory-raised Sceloporus undulatus from either Utah (grey line, triangles) or Oklahoma (dark line, squares). Dynamic Energy Budget model predictions are based on zoom factors (z) of 1 (Utah) and 0.92 (Oklahoma). b) Predicted and observed relationships between snout vent length (SVL) and body mass for the same two populations (Utah, grey; Oklahoma, black; observed relationship, solid line; predicted relationship, dotted lines). Data are from Ferguson and Talent (1993). Figure S7. Observed (dots) and predicted (lines) maximum and minimum available operative temperatures for Sceloporus merriami (slightly smaller than S. undulatus) at Big Bend National Park in Texas. Observed data come from (Grant & Dunham 1988) and are based on means from 12 or more measurements from painted copper models during late June and mid July during clear Metabolic theory, life history, and the distribution of a terrestrial ectotherm Michael Kearney Functional Ecology days. Predictions are from the biophysical model (Niche Mapper) based on the global dataset of long-term climatic averages for the point nearest to the study site, for clear skies in mid July. Figure S8. Predicted trajectories of wet body mass, reserve density and 12pm body temperatures for integrated biophysical/Dynamic Energy Budget simulations of Sceloporus undulatus at the 11 sites for which detailed life history data exists (see Table S1 for abbreviations of place names). Sites are in order of lowest to highest potential activity time. Predictions are based either on the Utah body size and clutch size (left column) or the locally observed body and clutch sizes (right column). Reserve density (J/cm3) has been divided by 100. Sudden drops in wet mass represent oviposition events, periods of low body temperature represent inactive times of the year due to cold (at which times the reserve density drops below the maximum level). Figure S9. Spatially explicit predictions of a) annual activity hours, b) lifetime clutches, and c) probability of survival to the end of year 3. All predictions are based on the estimated parameters for the Utah population. The solid black line represents the approximate rage limit within the USA while the filled triangles represent the sites for which detailed life history data are available, summarized in Tinkle and Dunham (1986). Figure S10. Predicted fraction of egg development for Sceloporus undulatus between June and September at two different depths in the soil, based on the following Arrhenius relationship derived from Parker and Andrews (2007): Tcor (temperature correction factor) = 1/(1+ EXP(TAL*(1/(273+Tsoil)-1/TL))+EXP(TAH(1/TH-1/(273+Tsoil)))); development per hour = k1*EXP(TA /T1-TA /(Tsoil +273))*Tcor*(1/24). Parameters were: TA = 9600, TAL = 50000, TAH = Metabolic theory, life history, and the distribution of a terrestrial ectotherm Michael Kearney Functional Ecology 90000, TL = 290, TH = 307.5, k1 = 0.0139, T1 = 298. Grey areas show where development would not complete. Figure S11. Predicted degree days of development for Sceloporus undulatus between June and September at two different depths in the soil, based on data from Parker and Andrews (2007). The lower critical temperature for degree day calculation was 17 °C. Parker and Andrews predicted the minimum heat sum for development to be approximately 495 degree days (grey colours therefore show where development would not complete.). Metabolic theory, life history, and the distribution of a terrestrial ectotherm Michael Kearney Functional Ecology REFERENCES Andrews R.M., Mathies T. & Warner D.A. (2000). Effect of incubation temperature on morphology, growth and survival of juvenile Sceloporus undulatus. Herpetological Monographs, 14, 420-431. Andrews R.M. & Pough H.F. (1985). Metabolism of squamate reptiles: allometric and ecological relationships. Physiological Zoology, 58, 214-231. Angilletta M.J. (2001a). Thermal and physiological constraints on energy assimilation in a widespread lizard (Sceloporus undulatus). Ecology, 82, 3044-3056. Angilletta M.J. (2001b). Variation in metabolic rate between populations of a geographically widespread lizard. Physiological and Biochemical Zoology, 74, 11-21. Buckley L.B. (2008). Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. The American Naturalist, 171, E1-E19. Dawson W.R. & Bartholomew G.A. (1956). Relation of oxygen consumption to body weight, temperature, and temperature acclimation in lizards Uta stansburiana and Sceloporus occidentalis. Physiological Zoology, 29, 40-51. Ferguson G.W. & Talent L.G. (1993). Life-history traits of the lizard Sceloporus undulatus from two populations raised in a common laboratory environment. Oecologia, 93, 88-94. Grant B.W. & Dunham A.E. (1988). Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology, 69, 167-176. Grant B.W. & Porter W.P. (1992). Modeling global macroclimatic constraints on ectotherm energy budgets. American Zoologist, 32, 154-178. Hughes J.L., Fitzpatrick L.C., Ferguson G.W. & Beitinger T.L. (1982). Oxygen consumption and temperature acclimation in the northern prairie swift Sceloporus undulatus garmani from Kansas. Comparative Biochemistry and Physiology Part A: Physiology, 71, 611-613. Metabolic theory, life history, and the distribution of a terrestrial ectotherm Michael Kearney Functional Ecology Kooijman S.A.L.M. (2010). Dynamic Energy Budget Theory for Metabolic Organisation. Cambridge University Press, Great Britain. Marion K.R. (1970). Temperature as the reproductive cue for the female fence lizard Sceloporus undulatus. Copeia, 1970, 562-564. Parker S. & Andrews R. (2007). Incubation temperature and phenotypic traits of Sceloporus undulatus: implications for the northern limits of distribution. Oecologia, 151, 218-231. Tinkle D.W. (1972). The dynamics of a Utah population of Sceloporus undulatus. Herpetologica, 28, 351-359. Tinkle D.W. & Ballinger R.E. (1972). Sceloporus undulatus: a study of the intraspecific comparative demography of a lizard. Ecology, 53, 570-584. Tinkle D.W. & Dunham A.E. (1986). Comparative life histories of two syntopic Sceloporine lizards. Copeia, 1986, 1-18. Vitt L.J. (1978). Caloric content of lizard and snake (Reptilia) eggs and bodies and the conversion of weight to caloric data. Journal of Herpetology, 12, 65-72. Metabolic theory, life history, and the distribution of a terrestrial ectotherm Michael Kearney