MACROMOLECULE
advertisement

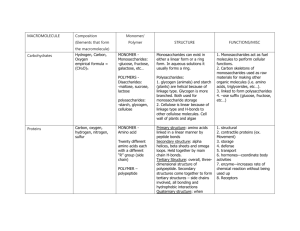
MACROMOLECULE Composition (Elements that form Monomer/ Polymer STRUCTURE FUNCTIONS/MISC the macromolecule) Carbohydrates Hydrogen, Carbon, Oxygen empirical formula = (CH2O) MONOMER Monosaccharides: -glucose, fructose, galactose, etc… Monosaccharides can exist in either a linear form or a ring form. In aqueous solutions it usually forms a ring. POLYMERS Disaccharides: -maltose, sucrose, lactose Polysaccharides: 1. glycogen (animals) and starch (plants) are helical because of linkage type. Glycogen is more branched. Both used for monosaccharide storage 2. Cellulose is linear because of linkage type and H-bonds to other cellulose molecules. Cell wall of plants and algae polysaccharides: -starch, glycogen, cellulose Proteins Carbon, oxygen, hydrogen, nitrogen, sulfur MONOMER Amino acid Twenty different amino acids each with a different “R” group (side chain) POLYMER – polypeptide Primary structure: amino acids linked in a linear manner by peptide bonds Secondary structure: alpha helices, beta sheets and omega loops. Held together by main chain H-bonds. Tertiary Structure: overall, threedimensional structure of polypeptide. Secondary structures come together to form tertiary structures – side chains involved, all bonding and hydrophobic interactions Quaternary structure: when 1. Monosaccharides act as fuel molecules to perform cellular functions. 2. Carbon skeletons of monosaccharides used as raw materials for making other organic molecules (i.e. amino acids, triglycerides, etc…). 3. linked to form polysaccharides 4. –ose suffix (glucose, fructose, etc…) 1. structural 2. contractile proteins (ex. Movement) 3. storage 4. defense 5. transport 6. hormones—coordinate body activities 7. enzyme—increases rate of chemical reaction without being used up 8. Receptors Nucleic Acids Hydrogen, oxygen, nitrogen, phosphate MONOMER – Nucleotide a. Sugar (deoxyribose in DNA or ribose in RNA), b. Phosphate, c. Nitrogenous base (A,G,C, T,U) POLYMERdinucleotide polynucleotide more than one polypeptide comes together, formed by bonding interactions among subunits (multiple polypeptides) NUCEOTIDE: A sugar, either deoxyribose or ribose depending on whether its DNA OR RNA, attached to a phosphate at carbon 5 and attached to a nitrogenous base at carbon 1 DINUCLEOTIDE: Two nucleotides connected by a phosphodiester linkage. C5-O-P-O-C3 POLYNUCLEOTIDE: Multiple nucleotides linked, sugar-phosphate backbone, 5’ and 3’ ends RNA: Single-stranded polynucleotide using A,U,C,G and ribose in nucleotides DNA: Double-stranded polynucleotide using A,T,G,C and deoxyribose in nucleiotides, stranded attached through basepairs A-T and C-G, A-T connected by 2 H-bonds, CG connected by 3 H-Bonds, weak H-bonds allow for DNA strands to be separated and read by RNA: Found in the steps between DNA and protein: 1. mRNA – a transcription of the DNA 2. tRNA – carries amino acids to the ribosome 3. rRNA – forms 60% of the ribosome DNA: Makes up genetic material that organisms get from parents. Stores RNA and protein sequence instructions. Lipids Fats (Triglycerides): glycerol + three fatty acids (carbon, oxygen, hydrogen) Phospholipids: a glycerol (C,H,O), two fatty acids (C,H,O), phosphate (P,O) and a variable group. Steroids : carbon, hydrogen, oxygen No monomers or polymers. proteins Fats: A glycerol with three fatty acids sticking out like tails. Phospholipids: A glycerol with two fatty acids ester linked (tails). The third site is attached to a negative phosphate, which is attached to a variable group. Steroid: Four fused carbon rings: one five-sided, three six-sided. Fats: energy storage, insulation, cushions organs. Phospholipids: a major component of cell membranes, form a lipid bilayer Steroids: cholesterol – part of cell membranes, helps maintain fluidity (prevents phospholipids from packing tight and becoming a solid), can be modified by enzymes to form the sex hormones (testosterone, progesterone, estrogen)