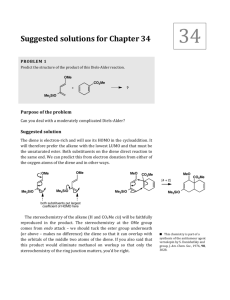
sujet - ISCE-Chem
advertisement

Professor Philip Page University of East Anglia at Norwich Program Interreg IS:CE Caen, 26-30 October 2009 "Synthesis of complex natural products" Problems The following scheme shows the synthesis of a key intermediate for prostaglandin synthesis by Corey. Cl H3CO H3CO CN OCH3 KOH Cl step 1 step 2 O CN m-CPBA step 3 O O O H3CO HO I2/KI OCH3 I step 5 OH (a) NaHCO3/H2O OCH3 step 4 O O OH Step 1 involves a Diels-Alder reaction. With the aid of diagrams, show the mechanism and explain the stereochemistry observed. (b) Indicate the mechanism of step 3 and explain the observed regiochemistry. (c) Step 5 involves an iodolactonization reaction. Indicate the mechanism using diagrams, and account for the observed regiochemistry and stereochemistry. The following scheme is taken from the synthesis of hirsutene by Curran. O O PhSe 1. LDA, thf, –78 °C PhSeCl O 2. t-BuMe2SiCl 3. heat step 1 OSi O O CH2Cl2 step 2 H step 3 THPO THPO CO2H H Li O O step 4 H H I H Bu3SnH AIBN, heat H (a) H step 5 H H Step 1 involves a sequence of reactions. Provide a mechanism for the heat-induced rearrangement reaction. Indicate the type of rearrangement involved, and the structural feature required for such rearrangements to take place. (b) Provide a mechanism for the reaction that takes place in step 2, and explain the stereochemistry observed. (c) Suggest a reagent for the reaction shown in step 3, and provide a mechanism for the reaction. Indicate the type of rearrangement involved. (d) Provide a mechanism for the reaction that takes place in step 4, and explain the stereochemistry observed. Suggest a competing process that could occur. (e) Suggest a mechanism for the reaction that takes place in step 5. The following scheme is taken from the synthesis of compactin by Grieco. R CO2Me MeO2C O H R MeO2C 1. MCPBA H R OH 2. MeOH 125 °C step 1 O step 2 O PhS SPh step 3 1. Ph3P; DEAD; PhCO2H 2. NaOMe, MeOH HO H R LiAlH4 MeO2C H R 1. Ac2O, base 2. Me2CuLi step 4 step 5 O O MeO2C H R OH O step 6 KH, toluene, 110 °C DEAD = CH3CH2OC(O)N=NC(O)OCH2CH3 OH (a) R Explain with the aid of a diagram the stereochemistry observed in the Diels-Alder reaction in step 1. (b) Step 2 involves a sequence of reactions. Provide a mechanism for the rearrangement reaction, indicate the type of rearrangement, and explain the stereochemistry observed. The rearrangement is an equilibrium process; indicate how the equilibrium is driven to give entirely the desired product. (c) Provide a mechanism for the reaction shown in step 3. (d) Explain why a cuprate reagent is used in step 4 rather than another type of organometallic reagent. (e) Provide a mechanism for the reaction shown in step 6. The following scheme is taken from the synthesis of oestrone by Oppolzer. O 1. CuLi 2. Br OSiR3 O 2 CO2Me HO MeO2C step 1 steps 2-4 steps 5-6 OSiR3 OSiR3 SO2 I NC step 7 SO2 NC heat step 8 OSiR3 OH 1. MeLi H H H 2. H3O H NC H 3. CF3CO3H 4. H3O H HO steps 9-12 (a) Explain the stereochemistry observed in step 1. (b) Suggest reagents that could be used for the transformation shown in steps 2-4. Note that R3SI = t- BuMe2Si (c) The anionic reagent shown in step 7 is generated by deprotonation of the parent sulfone with a strong base. Suggest a suitable base and explain the regiochemistry of deprotonation. (d) Explain the processes occurring in step 8, show the mechanisms, and provide an explanation for the observed stereochemistry. (e) Explain the reactions taking place in steps 9-12. The following scheme is taken from the synthesis of prostaglandin F2a by Stork. OMe O OH CH3C(OMe)3 O O O O 1 O Heat, H+ step 1 O O O O O step 2 MeO2C MeO2C 1. Base 2. TsCl (1 equiv) OTs O O O step 3 OH O O O O MeO2C MeO2C H+/H2O O O O HO step 4 O OH OH step 5 O O HO (a) OH Compound 1 was derived from D-glucose. What was the reason for incorporating the two different types of diol protection in this molecule ? . (b) Suggest a mechanism for the generation of the methyl enol ether shown in step 1 (c) Step 2 is an orthoester Claisen rearrangement. Explain the observed stereocontrol (d) Suggest a mechanism for removal of the carbonate protecting group shown in step 3 using hydroxide anion. (e) Give a mechanism for the hydrolysis of the cyclic acetal shown in step 4. (f) In step 5, there are three hydroxy groups that could react with the ester unit to provide a lactone product. Suggest why only one product is observed. The following scheme is taken from the synthesis of cholesterol by Woodward. O O O NaOH heat step 1 MeO step 2 MeO O MeO H O O H step 3 OH H+/H2O Ac2O/py O step 5 H OAc Zn step 4 O MeO H OH H OH step 6 NaOMe HCO2Et O step 7 step 8 O H O OHC H HO H O KOH step 9 H H O (a) Draw a transition state for the Diels-Alder reaction in step 1 and explain the observed stereocontrol. (b) Suggest a reagent to achieve the carbonyl group reductions shown in step 3. (c) Provide a mechanism for the reaction shown in step 4. (d) Suggest a mechanism for the reductive cleavage shown in step 6. (e) The reaction shown in step 7 is a Claisen condensation. Give a mechanism and explain the purpose of including this step in the synthesis. (f) Suggest reagents for the reaction shown in step 8 (g) Give mechanisms for the transformation shown in step 9 The following scheme is taken from the synthesis of reserpine by Woodward. O O O OH H H heat reduction step 1 step 2 CO2Me O H O CO2Me H O Br2 O O H Br O H H NBS/H2O HO OMe H MeO step 5 OMe H O step 4 O Br H O O oxidation step 3 O O step 6 O H Br H OH H OMe Zn/AcOH O OMe H O step 7 O O O (a) O Explain with the aid of a diagram the stereochemistry observed in the Diels-Alder reaction in step 1. (b) The carbonyl group reductions shown in step 2 are carried out using a hydride equivalent. Explain the stereochemistry observed. Indicate the product of the alternative lactonization process, and indicate why this does not occur. (c) Give a mechanism for the reaction shown in step 3, and explain the stereochemistry and regiochemistry observed. How is this relevant to the reaction shown in step 5 ? (d) Explain with the aid of a reaction mechanism why retention of stereochemistry is observed in step 4. (e) Provide a mechanism for the reaction shown in step 7. PG Problems: Sulfur and Related Chemistry Suggest structures for the major products the following reactions. Identify any intermediates. Explain the mechanism in each case and comment on any expected regioselectivity and stereoselectivity. O N O SPh N R HO O Bu3P H BuLi, –78 C, TMEDA; R S Ph R S Ph MeI BuLi; warm; MeI DMSO R Br NaHCO3 O– S+ Ph Ac2O CO2H OSiMe3 O– S+ R H TMSOTf + iPr2NEt, –78 C Ph O– S+ ² N B A O CO2Me OH 1. BuLi 2. PhSCl A B 1. LDA 2. MeI C D (MeO)3P MeOH OH PG Problems 2: Sulfur and Related Chemistry continued H KOtBu H SO2 Br THF, –15 °C SO2Ph 1. BuLi MsCl A 2.MeO2C Na/Hg B H O Base; SO2Ph CO2Me CO2Me OSitBuMe2 ² 213 C SO2 NC O O Me2S+ CH2– ? indicate products from both reactions Me2S+ CH2– ? O 1. base (2 equiv.) 2. Me3SiCl NNHTs OH OH 1. (imid)2CS 2. R3P or (MeO)3P C PG Problems: Silicon and Related Chemistry Suggest structures for the major products the following reactions. Identify any intermediates. Explain the mechanism in each case and comment on any expected regioselectivity and stereoselectivity. SiMe3 SnCl4 MeO OMe what principles of Si chem are illustrated here ? 1. BuLi SiMe3 2. RX what principle of Si chem is illustrated here ? SiMe3 R 1. mCPBA 2. ² SPh what principle of Si chem is illustrated here ? SiMe3 KH BF3.Et2O OH 1. Me3Si MgCl 2. AcOH O H C6H13 SiMe3 OsO4 MeOH 1. TsNHNH2 2. • 2 equiv BuLi 3. Me3SiCl O why are 3 equiv sometimes needed ? PG Problems 4: Silicon and Related Chemistry continued C6H13 SiMe3 1. DIBAL 2. MeLi 3. MeI CO2H SiMe3 Br2 PhCOCl AlCl3 SiMe3 Br2 Ph SiMe3 explain the observed double bond stereochemistry R1 R2 O TiCl4 SiMe3 OR Cl + AlCl3 O –60 C SiMe3 why not use a Grignard reagent ? Me3Si O Br2 Cl –70 C Cl CO2H CO2H SiMe3 peracetic acid Et2O, rt