Addition Reaction Mechanisms
Addition Reaction Mechanisms
Major concepts
The Electrophilic Addition mechanism involves a relatively weak nucleophile (a pi bond) reacting with a strong electrophile (usually a strong acid).
Multistep reaction mechanisms occur when intermediates are formed in the reaction pathway.
Based on the reactivity of a molecule, a mechanism of reaction can be predicted.
Based on a mechanism, the products of a reaction can be predicted.
The stereochemistry and regiochemistry of a product are determined by the reaction mechanism.
Vocabulary
Electophilic Addition
Mechanism intermediate
carbocation
Stereochemistry
Regiochemistry
Markovnicov product
Students should be able to:
Draw an arrow mechanism for an electrophilic reaction involving a strong acid or strong acid in water/alcohol
Explain how reactions of strong acids with alkenes are mechanistically related
Recognize addition reactions based on starting material and products
Predict the major products of an Electrophilic addition reaction, including proper stereochemistry and regiochemistry
Provide reagents necessary to produce the given products
Daily Problems
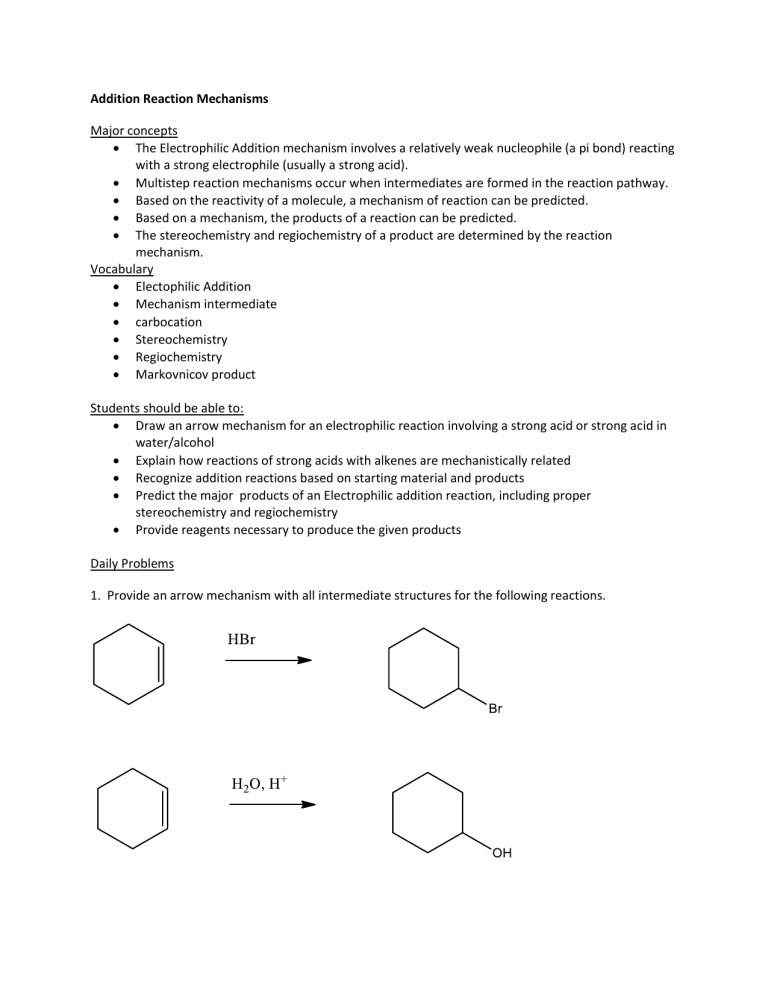
1. Provide an arrow mechanism with all intermediate structures for the following reactions.
2. Predict the major products, including stereochemistry, for the following addition reactions.
HCl
H
2
O, H
+
HBr
3. Which of the given products is the Markovnicov product? Explain.
4. Provide the necessary reagents.
5. In an addition reactions we have studied, the intermediate is a carbocation. How does the structure of the carbocation affect the stereochemical outcome of the product?
Cumulative problems
6. Predict the products when trans-but-2-ene is treated with HCl and when cis-but-2-ene is treated with
HCl. Are they the same or different? Why?
7. Treating 2-methylbut-2-ene with HCl yields 2-chloro-2-methylbutane. What happens if the same reaction is carried out with ethanol as the solvent? (Hint: What happens when you add HCl to ethanol solvent?)
8. When treating a diene with HCl, only one potential intermediate cation is formed, and not the other.
Explain.
HCl not formed
Once this intermediate is formed, it can lead to two products. Explain, and give a mechanism.
HCl
Cl
Cl
9. Are any of these reactions addition reactions?
O glutamate
-OOC
-OOC
O-
H
NH
2
O
H
O-
-2
O
3
PO
-2
O
3
PO
Vitamin B6
O
N
H
O fumarase
O-
-O -O
N
H
N
OH
O
O-
O-
O-
O O
COO
-
HC OPO
3
-2
OH H
2
C enolase
COO
-
C OPO
3
-2
CH
2
10. Some disease-causing bacteria have enzymes not found in humans that are used to produce branched carboxylic acids for incorporation into the cell wall. The enzyme catalyzes a reaction between
S-adenosyl methionine, an unsaturated carboxylic acid, and water according to this scheme:
O
O-
R
H
3
C OH
O
OOC S
R
O-
O
A
NH
3
OH
H
2
O
Propose a mechanism for this reaction based on these hints and information:
A. Compare to a reaction you know. What type of reaction is this?
B. The mechanism is multiple steps with a carbocation intermediate.
Extension problem
11. Draw an arrow mechanism for the reaction of propene with HCl. Compare the stability of the starting materials, the intermediates, and the products. Which are most stable? Which are least stable?











