7.9 Stereochemistry of Chemical Reactions
advertisement
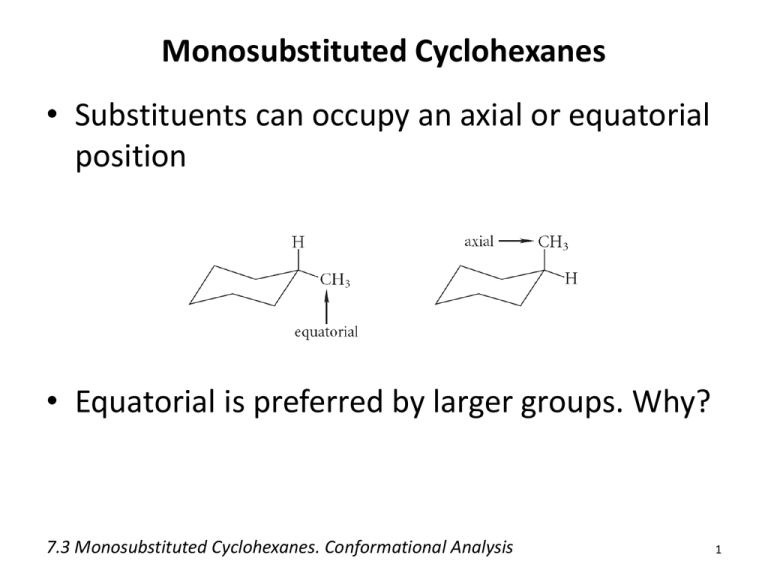
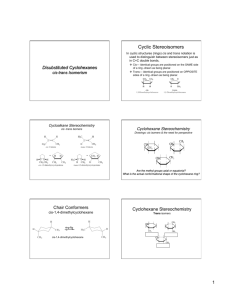
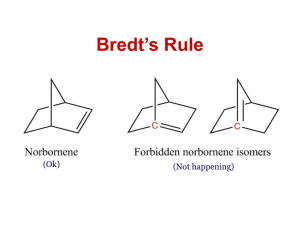
Monosubstituted Cyclohexanes • Substituents can occupy an axial or equatorial position • Equatorial is preferred by larger groups. Why? 7.3 Monosubstituted Cyclohexanes. Conformational Analysis 1 1,3-Diaxial Interactions • 1,3-Diaxial Interactions: van der Waals repulsions between an axial substituents on a cycloalkane ring 2 4 Problems 1) What is the energy cost of a 1,3-diaxial strain in methylcyclohexane? 2) What about bromocyclohexane? 3) Which of the two molecules above will have a higher percentage of its molecules in the equatorial conformation? More on Ring Flip • Note that the “up” substituent remains up and the “down” substituent remains down after chair interconversion 7 Disubstituted Cyclohexanes 1-chloro-2-methylcyclohexane • Trans: the two groups have an up-down relationship • Cis: the two groups have a down-down (or upup) relationship 8 Conformational Analysis • For disubstituted derivatives, the larger group will preferentially occupy the equatorial position 7.4 Disubstituted Cyclohexanes 9 Problem 1) Which subsituent will most likely occupy the equatorial position for a greater amount of time in cis-1-chloro-2-methylcyclohexane? 2) Prove this with calculation. Polycyclic Molecules • Polycyclic molecules: compounds with two or more rings fused together – Spirocyclic: two rings that have only a single common atom – Bicyclic: two rings that share two or more common atoms 11 7.6 Bicyclic and Polycyclic Compounds 12 Classification and Nomenclature • Fused and bridged bicyclic compounds 7.6 Bicyclic and Polycyclic Compounds 13 Naming Bicyclic Systems • Indicate the number of rings using the prefix “bicyclo-” • Indicate the bridge lengths – Number of atoms connecting one bridgehead atom to another (excluding the bridgehead atoms) – Separate by full periods and place in square brackets. • Cited in decreasing order of size (e.g. [3.2.1]) • the name of the hydrocarbon indicating the total number of skeletal atoms Problems • Name the following polycyclic molecules: Norbornane Decalin • If you were just given the IUPAC names for a couple of bicyclic molecules, how would you tell the difference between a fused and a bridged variety? Cis and Trans Ring Fusion: Decalin 7.6 Bicyclic and Polycyclic Compounds 16 • Each ring in cis-decalin can undergo ring flip • Ring fusion causes trans-decalin to be conformationally locked 17 Ring Fusion with Small Rings • Bicyclic compounds with small rings are restricted to cis ring fusion • Trans fusion would incur too much ring strain 7.6 Bicyclic and Polycyclic Compounds 18 Steroids • Organic compounds with 20 carbon, tetracyclic core – Variations in substituents dictate biological activity 7.6 Bicyclic and Polycyclic Compounds 19 Steroids • Many consist of all trans-fused rings – No conformational change • Many have methyl groups at C-10 and C-13 7.6 Bicyclic and Polycyclic Compounds 20 Stereochemistry of Cycloalkene Reactions Addition Reactions • Syn-addition: • Anti-addition: 7.9 Stereochemistry of Chemical Reactions 22 Stereochemistry of Bromine Addition • Addition of bromine to an alkene is a highly stereoselective reaction • Exclusively anti-addition – Gives all trans products 7.9 Stereochemistry of Chemical Reactions 23 Stereochemistry of Halohydrin Formation • Exclusively anti-addition – Gives all trans products Stereochemistry of Hydroboration-Oxidation • Hydroboration is a stereospecific syn-addition 7.9 Stereochemistry of Chemical Reactions 26 Stereochemistry of Hydroboration-Oxidation • The oxidation of organoboranes is a stereospecific substitution reaction 7.9 Stereochemistry of Chemical Reactions 28 Stereochemistry of Hydroboration-Oxidation • The two steps of hydroboration-oxidation result in net syn-addition of H-OH to the alkene • Note that the trans designation of the name has nothing to do with the way H-OH added 7.9 Stereochemistry of Chemical Reactions 29 Stereochemistry of Hydrogenation • Catalytic hydrogenation is a stereospecific synaddition 7.9 Stereochemistry of Chemical Reactions 30 Problem • Draw the products for the following reactions