1437528885Pro_Science_manuscript__new
advertisement

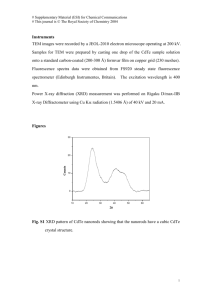
Easiest synthesis procedure of GeO2 nano-rod and its characterization Mrinal Seal1 Sampad Mukherjee2 Department of physics, Indian Institute of Engineering Science and Technology, Howrah, India Pin711103 Email-id: mrinal.phy@gmail.com Department of physics, Indian Institute of Engineering Science and Technology, Howrah, India Pin711103 Email-id: smukherjee.besu@gmail.com ABSTRACT horizontal tube furnace [7]. Bulk-quantity GeO2 nanorods and nanotubes have been synthesized by simple thermal evaporation of metallic germanium powders [8]. However, it is established for Zinc Oxide that the shape of the nano particles produced through solvothermal technique can be controlled by changing the solvent [9]. The presence of organic solvent in the solvothermal process creates mainly a mixture of sphere and rod like structure [10]. In this communication, we have followed the technique of using a mixture of organic solvent and water in hydrothermal process and report a novel as well as an economical synthesis procedure of GeO2 nanorods. Prepared sample is characterized with various techniques and compared with the results as obtained for the GeO2 nano crystals. Germanium oxide nanorods have been prepared hydrothermally using a mixture of water and acetone as organic solvent. The XRD pattern shows a new peak and PL spectrum reveals presence of a blue emission respectively as characteristic of the rod shaped sample. The SEM and HRTEM images confirm the rod shaped structure of the sample. The EDX pattern is a confirmation for the constituents of the rods are Germanium and Oxygen only. Finally, the magnetic behaviour of the sample is studied with the help of SQUID magnetometer and explained the origin of such behaviour with the help of oxygen vacancy. Keywords- Oxide, Hydrothermal Synthesis, Nanorod, Optical property, Magnetic property 2. EXPERIMENTAL PROCEDURE Analytical grade GeO2 powder (Alfa Aesar, 99.99%) is used to prepare a stock solution with moderately hot double distilled water. 40ml of this solution is taken in an in-house designed autoclave where 4 ml of acetone (organic solvent) is added. After sealing properly, the autoclave is heated in a furnace at temperature 3600C corresponding to 170 bar pressures as measured with a pressure gauge attached with the autoclave for twenty-four hours. After that, the autoclave is cooled naturally to ambient temperature and the samples are collected from the solution by evaporating water. Collected samples were grinded with mortar and pestle and prepared for different types of characterization. 1. INTRODUCTION Germanium oxide (GeO2) is an important material due to its wide band gap and high refractive index leading to possible applications in opto-electronic devices. Such opto-electronic applications need GeO2 mostly in rod or wire shaped form. There are so many reports of GeO2 nano wire synthesis using different techniques like thermal evaporation [1], Carbon nano-tube confined reaction of Germanium [2], laser ablation [3], thermal oxidation [4], carbothermal reduction [5] etc. All these techniques have performed at the temperature range of 820-13000C to generate sufficient vapour pressure of Germanium (Ge) or germanium mono oxide (GeO) in an oxidizing or inert environment to prepare GeO2 nanowires or whiskers. The GeO2 nanowires were also synthesized utilizing the vapourliquid-solid (VLS) growth mechanism [6] in a simple The X-ray diffraction (XRD) pattern is obtained using Mini Flex2 X-ray powder diffractometer with Cukα (wave length-1.54Ǻ) radiation and operating in a step scan mode with 0.010 in 2θ per step. High- 1 resolution Transmission Electron Microscope (HRTEM) (TECNEI20) photographs are obtained after preparing the grids following conventional methods. Scanning Electron Microscope (SEM) image as well as the Energy Dispersive X-ray (EDX) pattern of the same also obtained with Hitachi (S3400N) microscope. Photo luminescence (PL) spectroscopy is performed with Spectrophotometer (Perkin Elmer Model: Lambda45) at different excitation energies. The prepared nano rods show some unusual features regarding magnetization effect as observed through SQUID magnetometer (magnetic property measurement system, Quantum Design). 3. RESULTS AND DISCUSSIONS Figure 1 shows the XRD pattern of the sample. The peaks as indicated with hkl values are in fair agreement with the XRD pattern obtained for αquartz GeO2 nano crystals as prepared by hydrothermal technique [11-12], except the peak nearly 310 as marked with a circle. Fig.2. SEM image of GeO2 nanorods. Fig.3. HRTEM image of GeO2 nano rods. Fig.1. XRD Pattern of GeO2 nanorods. From the SEM image it is observed that rod shaped structures appears with the usual GeO2 crystal forms [11], which is comparable to the phenomenon as occurs due to use of organic solvent [10]. The length of the rod shaped sample as observed from the SEM image is of the order of a few micrometers and the diameter as observed from the TEM image is measured nearly 100 nm. EDX spectrum as shown in figure 4 confirms existence of germanium and oxygen only inside the sample. This XRD pattern with such peak is very much similar to the XRD pattern of rod shaped GeO2 as prepared earlier [8] with different technique. The figures 2 and 3 show the SEM and HRTEM images respectively. 2 Fig.4. EDX pattern of GeO2 nano rods. The PL spectrum of the prepared GeO2 nanorods for excitation wavelength 300 nm is presented in figure 5. Foremost, it is being observed an emission peak around 420 nm (Position-a, as marked with solid straight line in figure 5). Such peak in the blue light region for nano-crystal GeO2 particles are due to presence of defects including oxygen vacancy [13, 5]. The peak as observed nearly at 450 nm (Position-b, as marked with dotted straight line in figure 5) is due to presence of oxygen Fig.6. Hysteresis curves of the sample at 295K and 2K respectively. The figure shows that the area covered by the hysteresis loop is greater at lower temperature for the sample, which is obvious. However, ferromagnetism due to oxygen vacancy in some oxides (viz. SnO2, CeO2 especially in nano-order phase) is already reported [14, 15]. The report by Chang et al [14] illustrated that the sample (nano sized SnO2) loses its ferromagnetic nature due to absence of Oxygen vacancy as the material is annealed at 7000C in oxygen atmosphere. From the study concerning the magnetic behavior of such samples, it is clear that oxygen vacancy plays an important role for exhibiting the property of magnetization. A schematic diagram of GeO2 crystal structure is shown in figure 7, where the black and grey coloured spheres are denoted as Germanium and Oxygen respectively. Fig.5. PL Spectrum of GeO2 nano rods excitation energy 300nm. vacancy in the rod-shaped sample and comparable with the PL spectrum as observed by Z. Jiang et al.[8]. The most unusual property of the sample, contradictory to the bulk or crystalline GeO2, is the magnetization effect. Although the amount of magnetization is not considerable at room temperature but still a hysteresis curve is obtained for the sample as placed within high magnetic field. Figure 6 shows the hysteresis curve as obtained with SQUID magnetometer at both room temperature (295K) and at 2K. Fig.7. Schematic diagram of GeO2 molecules for rod shaped structure. Regarding the electronic configurations of Germanium and Oxygen it must be that, each electron of 4p shell of Germanium is coupled with another electron of 2p shell of an Oxygen atom with exchange interaction. However, from the PL study 3 we have evidence of defects in the sample. Such defects inside the sample indicate formation of oxygen vacancies, which is responsible for the partially filled 4P shell in Germanium. This idea has been adopted from the explanations given by Han et al for Oxygen-deficient Cerium Oxide [15] as the structure of the material is quite similar to GeO2. Such oxygen-vacancy in the sample creates unpaired electrons, which is responsible for the magnetic behavior of the sample. The responsibilities of oxygen-vacancy in magnetic and transport behaviour within a few oxides have also been established [1617]. REFERENCES [1] [2] [3] 4. CONCLUSIONS We have prepared GeO2 nano rod by hydrothermal technique using a mixture of water and acetone as solution. The use of such organic-solvent in solvothermal process to control the shape of the sample is an established one, which we have used for GeO2 rod preparation first time and we are successful. XRD pattern of such sample shows a peak that presents only for the rod shaped GeO 2 sample with the other conventional XRD pattern of GeO2 crystals. The elemental analysis of prepared sample is also done from the EDX pattern. Therefore, we suggest that the prepared sample is a mixture of rod and usual crystal form as confirmed from SEM image also. The XRD peak near 310 is a characteristic peak for the rod shaped GeO2 sample. The SEM and HRTEM images established that the length of produced GeO2 rods are of the order of a few micrometers and the diameter is nearly 100 nm respectively. The PL spectroscopy reveals that there exist defects inside the sample; besides this, a characteristic blue peak near 450 nm is also observed for such rod shaped sample. Such peak produced at low energy region indicates the increment of surface area due to formation of GeO2 nano rod (a part of the internal energy is used for the increment of surface area).The magnetic hysteresis curve as observed for the sample, both at room temperature (295K) and at 2k are the first ever reported for GeO2 and this property is certainly due to the presence of oxygenvacancy for the rod shaped sample. [4] [5] [6] [7] [8] [9] [10] ACKNOWLEDGEMENT We acknowledge IISER, Kolkata for the SQUID magnetometer measurement and for XRD pattern. We are thankful to the Material Science Department of IIEST, Shibpur for the SEM micrograph as well as for EDX. We also extend our thanks to IIEST, Shibpur for completion of this work. [11] 4 Dang H. Y., Wang J., and Fan S. S., 2003, The synthesis of metal oxide nanowires by directly heating metal samples in appropriate oxygen atmospheres, Nanotechnology,14, ( 7), pp 738-741. Zhang Y., Zhu J., Zhang Q., Yan Y., Wang N., Zhang X., 2000, Synthesis of GeO2 nanorods by carbon nanotubes template, Chem. Phys. Lett., 317, (3-5), pp 504-509. Tang Y. H., Zhang Y. F., Wang N., Bello I., Lee C. S., Lee S. T., 1999, Germanium dioxide whiskers synthesized by laser ablation, Appl. Phys. Lett., 74, pp 3824-3826. Wu C. I., Hogan T. P., 2006, Growth of GeO2 nanowires by thermal annealing, Mater. Res. Soc. Symp. Proc., 940. Wu X. C., Song W. H., Zhao B., Sun Y. P., Du J. J., 2001, Preparation and photoluminescence properties of crystalline GeO2 nanowires, Chem. Phys. Lett., 349, (34), pp 210-214. Wagner R. S., Ellis W. C., 1964, Vaporliquid-solid mechanism of single crystal growth, Appl. Phys. Lett., 4, pp 89. Khan M. A. , Farhan M. F., Hogan T. P., 2009, Low temperature synthesis of germanium oxide nanowires by thermal evaporation of germanium in an oxidizing environment , IEEE NM DC. Jiang Z., Xie T., Wang G.Z., Yuan X.Y., Ye C.H., Cai W.P., Meng G.W., Li G.H., Zhang L.D., 2005, GeO2 nanotubes and nanorods synthesized by vapor phase reactions, Mater. Lett., 59, (4), pp 416- 419. Khoza P.B., Moloto M. J., Sikhwivilu L. M ., 2012, The effect of solvents, acetone, water and ethanol on the morphological and optical properties of ZnO nanoparticles prepared by microwave, Journal of Nanotechnology, 2012, article Id. 195106. Xu L., Hu Y. L., Pelligra C., Chen C. H., Jin L., Huang H., Sithambaram S., Aindow M., Joesten R., Suib S.L., 2009, ZnO with different morphologies synthesized by solvothermal methods for enhanced photo catalytic activity, Chem. Mater., 21, (13), pp 2875–2885. Seal M., Mukherjee S., 2015, Approach for selection of a synthesis procedure of GeO2 ultra-small nanoparticles and its characterization, International Journal of Phys., 3, (3), pp 133-138. [12] [13] [14] [15] Bose N., Basu M., Mukherjee S., 2012, Study of optical properties of GeO2 nanaocrystals as synthesized by hydrothermal technique, Mater. Res. Bull.,47, ( 6), pp 1368-1373. Kim H.W., Lee J.W., Kebede M.A., Kim H.S., Lee C., 2009, Catalyst-free synthesis of GeO2 nanowires using the thermal heating of Ge powders, Curr. Appl. Phys., 9, (6), pp 1300-1303. Chang G. S., Forrest J., Z. Kurmaev E., Morozovska A. N., Glinchuk M. D., McLeod J. A., Moewes A., Surkova T. P., Hong N. H., 2012, Oxygen -vacancy induced ferromagnetism in undoped SnO2 thin films, Phys. Rev. B., 85, pp 165319. Han X., Lee J., Yoo H. I., 2009, Oxygenvacancy-induced ferromagnetism [16] [17] 5 in CeO2 from first principles, Phys. Rev. B., 79, pp 100403. Shah L. R., Ali B., Zhu H.,. Wang W. G, Song Y. Q., Zhang H. W., Shah S. I., Xiao J. Q., 2009, Detailed study on the role of oxygen vacancies in structural, magnetic and transport behaviour of magnetic insulator: Co–CeO2, J. Phys. Condens. Matter, 21, (48), pp 486004. Pan X., Yang M. Q., Fu X., Zhang N., Xu Y. J., 2013, Defective TiO2 with oxygen vacancies: synthesis, properties and photo catalytic applications, Nanoscale, 5, ( 9), pp 3601-3614.