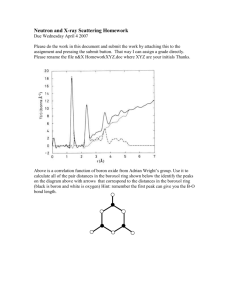
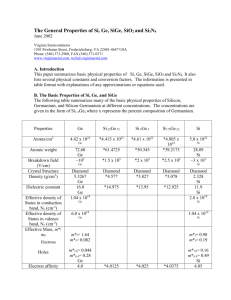
Germanium surface passivation using ozone gaseous phase
advertisement

Germanium surface passivation using ozone gaseous phase Virginie Loup1,a, Pascal Besson2,b, Olivier Pollet3, Eugénie Martinez1, Emmanuelle Richard4, Sandrine Lhostis2. 1 CEA-DRT - LETI/DPTS - CEA/GRE – 17 avenue des Martyrs, 38 054 Grenoble Cedex 9, France 2 STMicroelectronics, rue Jean Monet, F-38920 Crolles, France 3 SEMITOOL, 655 W. Reserve drive, 59901 Kalispell MT, USA 4 LTM/CNRS – 17 avenue des Martyrs, 38 054 Grenoble Cedex 9, France a virginie.loup@cea.fr, b pascal.besson@cea.fr Keywords: germanium, surface passivation, ozone. Introduction As silicon tends towards ultimate physical limitations for high-performance devices, germanium is extensively investigated for its high electron and hole mobility advantages [1]. As for silicon, advanced integration node implies the use of very efficient germanium cleaning sequences to gain yield. However, germanium surface sensitivity against most of the conventional wet solutions is a major issue [2]. Specific wet chemical processes also need to be developed to clean and prepare germanium surfaces before being integrated. In this study, the influence of various chemical treatments on germanium surfaces state is investigated using AR-XPS analysis. Then, a new method to efficiently passivate a germanium surface is described. Using gaseous ozone phase on a spin-on single wafer cleaning tool, we effectively managed to grow a GeO2 layer which is, according to AR-XPS analysis, stable in time. Experimental details In this work, 2.5 µm thick germanium layers epitaxially grown on Si (001) wafers by Chemical Vapor Deposition were used [3]. Cleaning tests were performed on three different tools. The Akrion Gama One automated wet bench was mainly used to evaluate the compatibility of typical silicon wet treatments (CARO, SC1, SC2…) with germanium surfaces cleaning. A 200 mm spin-on single wafer tool from SEZ was also used for HF-last processes. Finally, both wet and vapor phase cleanings were experimented on the 200/300 mm Raider SP spin-on single wafer tool from Semitool. Post cleaning, germanium surfaces were analyzed by AR-XPS at various incidence angles, with a 0.7 eV energy resolution. The angle scanning enables to enhance the sensibility either to the extreme surface or to the interface with the substrate. Both Ge 3d and Ge 2p (respectively centered at 29 and 1217 eV) were also used to vary the scanned thickness according to the photoelectrons kinetic energy. For each measurement condition, analyzed thicknesses (d) can be estimated with the following formula: d = 3×L×sinθ, where θ represents the scanning angle and L, the electrons mean free path (in GeO2, L is around 2.35 nm and 0.6 nm respectively for Ge 3d and Ge 2p). Results and discussion As germanium is a potential candidate for the 45 nm node and below, its consumption control during cleaning steps is a key point. However, because of the high sensitivity of germanium to oxidation combined with the high kinetic of dissolution of GeO2 in water, standard H2O2-based chemistries (like CARO, SC1 and SC2) lead to excessive etch rates (see Figure 1 and Table 1). Consequently, standard H2O2-based wet chemical treatments are totally prohibited for germanium cleaning. Among the wet solutions classically used for silicon cleaning, only the HF/HCl solutions can potentially candidate for germanium treatments. However, even the so-called “DDC” cleaning process developed in LETI [4] leads to a 200 Å germanium consumption which is due to the acidic ozonated DIW rinse step necessary to guarantee a high particle and metal removal efficiency. Adapting the “DDC” process, a new sequence (called “GecleanP”) has been optimized for germanium surfaces cleaning applications. First, samples are des-oxidized in an acidic (HF 0.2% + HCl 1%) solution for 120 s to dissolve the native oxide. Next, germanium wafers are rinsed in a two-step sequence: first, in an acidic de-ionized water (DIW) solution (with a pH near to 2) for 180 s; and then in an acidic ozonated DIW solution (strict control of the O3 injection) for 120s. This two-step rinse sequence enables to combine cleaning efficiency (particle and metal removal > 98%) with a low Ge consumption (~35 Å). As supported by the XPS spectrum presented in Figure 2, following the “GecleanP” rinse step, the germanium surface is covered by a thin oxide (GeOx) layer. Compared with the GeO2 peak energy position, the GeOx peak is shifted slightly toward the lower binding energy values, indicating the under stoechiometry of this germanium oxide (x<2). Moreover, XPS spectra of germanium wafers rinsed in pure DIW (not shown here) show a similar GeOx peak signature. These results totally contrast with what happens on silicon surfaces: indeed, the XPS spectrum of a “DDC” cleaned Si substrate clearly differs from a “HF-last” Si surface in the presence or not of the Si-O peak signature. Consequently, a stable germanium interface cannot be obtained after a DIW rinse in presence of diluted ozone or not. In order to bypass a potential re-oxidation due to the DIW rinse, the rinse step was removed from the germanium cleaning sequence. XPS spectra are given in Figure 3. Even when XPS analysis immediately follows the HF/no rinse treatment, a shoulder is observed on the elemental germanium peak pointing out that the germanium first monolayers are instantaneously re-oxidized by air exposure only. After 48 hours storage in clean room, XPS analysis clearly demonstrates the increase and the shift of the GeOx-attributed peak indicating the re-growth of the GeOx layer. According to XPS quantifications (given in Table 2), wet processes (with or without rinse) inevitably leave a non stabilized sub-oxides–covered germanium surface which gradually oxidizes, tending toward the GeO2 stoechiometry without reaching it. As stable and passivated germanium surfaces cannot be achieved by standard wet cleaning sequences, gas phase treatments, especially treatment including ozone, were investigated on the Raider SP tool. Among the nine process chambers available on this cleaning equipment, the specific configuration of the so-called “Spray” chamber was exploited. Initially developed for stripping using the FluorozoneTM process [5], HF liquid solution and ozone vapor can be simultaneously injected in the chamber. For specific germanium surface treatments, the liquid phase was suppressed from the recipe and concentrated gaseous phase ozone was directly injected on germanium wafers. XPS spectra corresponding to ozone-treated germanium substrates are presented in Figure 5. We can observe that the ozone-formed germanium oxide and the Ge fundamental peaks are separated by a 3.5 eV binding energy gap. In other words, using an ozone gaseous phase treatment enables to grow a stoechiometric GeO2 passivation layer. According to ellipsometry measurements, the GeO2 layer thickness can be estimated to about 20 Å. This value is corroborated by XPS data. Varying the XPS scanning angle and so the scanned depth, we can distinguish the germanium states respectively present near the oxide surface and near the germanium substrate interface. For this study, the Ge 2p line is more adapted than the Ge 3d one. Indeed, thanks to a lower photoelectrons kinetic energy (Ec = 269 eV compared with 1457 eV for Ge 3d), the Ge 2p line is more sensitive to the sample’s extreme surface. The XPS extracted data (Figure 6) highlight the presence of both GeOx sub-oxides and GeO2 components. AR-XPS also shows that the GeO2 component is located on the surface (GeO2 contribution systematically higher at 10°) whereas the germanium sub-oxides are more localized near the germanium interface. To conclude, XPS analysis tend to prove that the ozone-formed germanium oxide is in fact constituted by a Ge substrate/GeOx/GeO2 stack, in accordance with what is well-known for silicon oxide. The stability of the gaseous phase passivation was also checked. Ozone-treated germanium wafers were stored in clean room during one month. Totally oxidized, this germanium oxide is stable after one month ambient air exposure but, not surprisingly, very unstable in water (see Figure 7). Indeed, upon water immersion, only GeO2 disappears confirming that the stoechiometric GeO2 is more soluble than the GeOx sub-oxides [2]. Conclusion Using an ozone gas-based process, we managed to passivate germanium surfaces with a stable, smooth and stoechiometric GeO2 layer. Unlike “native” re-oxidation by air exposure following wet cleaning, the proposed method for germanium passivation is well-controlled, reproducible, industrially viable and compatible with the criteria of minimal germanium consumption. References [1] C. Chui et al., IEDM Dig. (2002), p 437-438. [2] B. Onsia, Proceeding of the Int. Symp., UCPSS, Belgium (2000). [3] J.M. Hartmann , J. Appl. Phys. 95, (2004), p 5905. [4] F. Tardif, Proceeding of the Int. Symp., UCPSS, Belgium (1996), p 175. [5] 10000 SC10.02/1/20 @65°C EDI @ 60°C Etch Rate [Å/min] 1000 EDI @ RT + O3 + HCl SC2 1/2/20 @ 50°C CA RO @ 110°C 100 10 1 0,1 0 20 40 60 80 100 [%Ge] Figure 1: SiGe alloys (0< [%Ge] <100) etch rates in standard cleaning solutions. Cleaning process Ge etch rate HF 0.5% @ RT 1.5 Å/min DIW @ RT (+N2) 0.5 Å/min O3 solution @ RT 70 Å/min Table 1 : Germanium etch rates in diluted HF chemistry, DIW and Ozone solution. GeOx GeO2 Ge-Ge Figure 2: XPS spectrum of a germanium surface after a DIW rinse. Ge po st DIW rinse Ge 3d - 10° Ge po st HF witho ut rinse (t0) Ge po st HF witho ut rinse (t1) 36 34 32 30 28 26 Binding energy (eV) Figure 3: Compared XPS spectra (at grazing incidence) for a DIW rinsed Ge sample and two “HF- last” Ge surfaces respectively immediately (t0) and one week post treatment (t1) Ge0 % Sample Ge post DIW i rinse Ge post HF (t1) Ge post HF (t0) GeOx % 1+ 2+ 3+ 20,7 1,8 2,7 7,1 GeOx total % 11,6 17,8 26,4 2,8 4,1 1,2 2,9 8,0 4,7 12,0 11,7 GeO2 % 4,0 - 1,6 Table 2: Atomic concentrations (%) of GeOx and GeO2 extracted from Ge 3d spectra (Analysis angle = 10°). Comparison of Ge oxidations states between a rinsed only Ge and Ge surfaces just after (t0) or one week after (t1) HF treatment (without rinse). GeOx Ge- Ge GeO2 Figure 5: XPS spectrum of a germanium surface passivated by the ozone vapor process. 1,2 Relative intensities GeO2 GeOx Ge-Ge 1 0,8 0,6 0,4 0,2 0 2p (10°) 2p (35°) 3d (10°) 3d (35°) Figure 6: Ge0, GeOx and GeO2 relative intensities for a ozone passivated Ge surface (scanning angles = 10° and 35°). 120 Atomic concentration (%) Ge O C 100 80 60 40 20 0 P2-t0 P2-t1 P3-t0 P3-t1 Figure 7: XPS extracted concentrations respectively at t0 (immediately post treatment) and t1 (one month‘s wait). P2 corresponds to a ozone passivated Ge surface and P3 to a ozone passivated Ge surface after DIW rinse.