rcm_4285_sm_suppInfo
advertisement

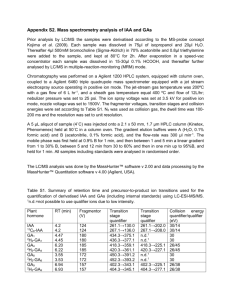
ON LINE SUPPORTING INFORMATION Use of ion mobility mass spectrometry and a collision cross-section algorithm to study an organometallic ruthenium anticancer complex and its adducts with a DNA oligonucleotide Jonathan P. Williams1*, Julie A. Lough1, Iain Campuzano2, Keith Richardson2 and Peter J. Sadler1* 1 Department of Chemistry, University of Warwick, Coventry CV4 7AL, UK 2 Waters Corporation, Floats Rd, Manchester M23 9LZ, UK *Correspondence to: J. P. Williams or P. J. Sadler, Department of Chemistry, University of Warwick, Coventry CV4 7AL, UK. E-mail: J.P.Williams@warwick.ac.uk; P.J.Sadler@warwick.ac.uk 1 (S1) Materials The [(η6-biphenyl)Ru(N,N′-1,2-ethylenediamineCl].PF6 and [(η6-biphenyl)RuCl2]2 was prepared using the method previously described1 and references therein. Sodium chloride solutions used for hydrolysis suppression were 154–155 mM and produced using sodium chloride purchased from Acros and deuterium oxide from Aldrich. 1,4Dioxane was purchased from Rathburn and RuCl3×H2O was purchased from Precious Metals Online (Australia). (S2) Ion mobility calculations using MOBCAL The MOBCAL output is based on three different models which can calculate the theoretical collisional cross section of any molecule possessing a three-dimensional coordinate file.2,3 The Projection Approximation (PA) models the ion as a collection of overlapping hard spheres with radii equal to hard sphere collision distances. The orientationally averaged geometric cross section is determined by averaging the geometric cross section over all possible collision geometries. Exact Hard Sphere Scattering (EHSS) models the ion as a collection of overlapping hard spheres with radii equal to hard-sphere collision distances. The orientationally averaged momentum transfer cross section is calculated by determining the scattering angles between the incoming buffer gas atom trajectory and the departing buffer gas atom trajectory. The Trajectory Method (TM) models the ion as a collection of atoms, each one represented by a 12-6-4 potential. The effective potential is obtained by summing over the individual atomic contributions. Trajectories are run in this potential to obtain the scattering angle (the angle between the incoming and departing buffer gas atom trajectory). The orientationally averaged collision integral is determined by averaging over all possible collision geometries. 2 (S3) Ion mobility principles and the calculation of collision cross-section measurements using the T-Wave based ion mobility device In brief, traditional drift tube ion mobility systems measure the time taken for ions to traverse a buffer gas-filled cell under the influence of a uniform electrostatic field (E). The mobility (K) of an ion is dependent on several factors including the masses of both the ion and the buffer gas, the charge on the ion and the rotationally averaged collision cross-section (ion-neutral collision cross section). The average drift velocity (vd) that an ion attains in the buffer gas as a result of the electric field is given by: vd = K E (1) Experimentally, K values can be obtained by measuring the time taken for a packet of ions to traverse a drift cell of known length and these values are used to determine the CCS (or Ω) values using the theoretically derived relationship:4 = ((18)1/2/16) (ze/(kbT)1/2) (1/mion + 1/mgas)1/2 1/(NK) (2) where, ze corresponds to the charge on the ion; kb is Boltzmann's constant; N is the number density of the neutral gas; the term (1/mion + 1/mgas) is the inverse of the reduced mass relating to the ion and neutral, with mion and mgas being their respective masses. The path ions take using the T-Wave mobility device is more complex than a conventional drift tube ion mobility system since the electric field is neither constant nor uniform. As a consequence, direct determination of mobilities from the measured drift times is not easy. The systematic approach taken here, as with previous studies, is to calibrate the T-Wave ion mobility device using species of known CCS determined using standard drift tubes. The previous studies have shown that calibration of the T-Wave device does indeed provide CCS values in good agreement 3 with those obtained from drift tube studies. The effective drift time is plotted against normalised cross section and a power or linear trendline of the form y = axb or y = ax + b fitted to the data. The effective drift time, derived coefficients of a and b, z (charge) and (reduced mass) were then used to calculate the collision cross sections of ions in the T-Wave device. [Note: The mass-dependent and mass-independent flight times were corrected for during this study. The mass-dependent flight time is the time from when the ion exits the transfer T-Wave until it reaches the detector and is proportional to the m/z of the ion. The mass-independent flight time is the time taken by an ion to traverse the mobility and transfer T-Wave cells and does not depend on the m/z of the ion.] 4 (S4) Validation of Waters collision cross-section algorithm 5 REFERENCES 1. Morris RE, Aird RE, Murdoch PS, Chen H, Cummings J, Hughes, ND, Parsons S, Parkin A, Boyd G, Jodrell, DI, Sadler PJ. J. Med. Chem. 2001; 44: 3616. 2. Mesleh M, Hunter J, Shvartsburg A, Schatz G, Jarrold MJ. J. Phys. Chem. 1996; 100: 16082. 3. Jarrold MJ. Available: http://wwwindiana.edu/~nano/Software/mobcaltxt. 4. Mason EA, McDaniel EW. Transport Properties of Ions in Gases, John Wiley: New York, 1988. 6