Ion naming and Ionic Bonding Notes
advertisement

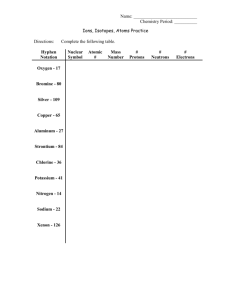
Name ______________________________________________________ Period ___________________ Chapter 7/8: Bonding Keller/Rosenzweig Video 7-8.1: Naming Ion Notes Answer these questions while watching the video: 1. Why do atoms form ions? (this is a question you’ve seen before and should be review!) 2. Create a rule for naming cations. Write it here: 3. Create a rule for naming anions. Write it here: Answer these questions after watching the video: 4. For the transition metals, the charge of the ion is given by the Roman Numeral in its name. Remember, these metals can have multiple charges so you must be told what the charge is on a case by case basis! Since transition metals are metals, they follow the same rule as the cations above! I-1 II - 2 III - 3 IV - 4 V-5 VI - 6 VII - 7 VIII - 8 IX - 9 X – 10 What is the charge of the following ions? copper (I) ion _____ cobalt (II) ion ______ tin (IV) ion ______ iron (III) ion _______ 5. For each of the elements listed below, write the charge of the ion it forms and name the ion according to the rule above. Element Ion Charge Sodium Fluorine Oxygen Beryllium Asenic Aluminum Manganese +2 Ion Name Class Notes: Forming Binary Compounds sodium chlorine magnesium 1. Write the formula for the following ionic compounds using the following rules: a. Identify and write in the charges of the cation and anion in the compound b. Criss-cross the charges c. Reduce subscripts if necessary a. lithium fluoride f. lead (II) nitride b. calcium bromide g. tin (IV) phosphide c. magnesium phosphide h. tin (IV) iodide d. barium oxide i. tin (IV) oxide e. lithium sulfide j. iron(III) chloride 2. Name the ionic compound using the following rules: a. Name the cation b. Name the anion a. Li2O b. MgF2 c. Ca3P2 d. SrI2