Field sites
advertisement

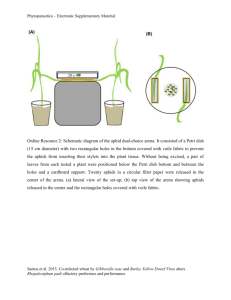
Ants defend aphids against lethal disease SUPPLEMENTARY MATERIAL Charlotte Nielsen, Anurag A. Agrawal & Ann E. Hajek Field sites Experiments were carried out at 3 field sites near Ithaca, New York, USA (N 42.44, W 76.50): in August 2005 on Niemi Road and at the Plant-Insect Interactions Field Lab on Freese Road and in July 2007 at Brooktondale Meadow. At the Plant-Insect Interactions Field Lab site, two naturally established milkweed plants (Asclepias syriaca) with Aphis asclepiadis colonies (11-66 individuals per colony) that were actively tended by 1-10 Formica podzolica workers were selected. The plants were growing approximately 5 m from the nearest F. podzolica nest. At the Niemi Road field site, 9 transplanted and 2 naturally established milkweed plants with A. asclepiadis colonies (8-151 individuals) that were actively tended by 1-10 F. podzolica workers were selected. The 9 transplanted plants were 1.5 m from the nearest F. podzolica nest and the remaining 2 plants were growing approximately 5 m from the nearest nest. At the 2007 site, 26 naturally established milkweed plants with A. asclepiadis colonies (13 - >400 individuals per colony) actively tended by 1-11 F. podzolica workers, were selected. The plants were growing < 5 m from the nearest F. podzolica nest. Aphis asclepiadis Aphids used as test insects originated either from naturally established colonies in the field or laboratory colonies of A. asclepiadis originally collected from the field near Ithaca. Aphids were maintained in the laboratory on potted milkweed plants or, for short periods of time, on cut milkweed leaves. All aphids were kept at room temperature until use for production of experimental aphids. Experimental aphids were produced every day. Production of experimental aphids Sporulating cadavers: Aphids became infected after exposure to conidia from a sporulating in vitro culture of P. neoaphidis (ARSEF 7214; USDA Agricultural Research Service Collection of Entomopathogenic Fungal Cultures, Ithaca, NY) isolated from Aphis glycines collected in NY, USA in 2003. The actively ejected conidia were obtained from a fungal mat as described by Papierok & Hajek (1997). Healthy apterous adults and 4th instar nymphs were transferred to a milkweed leaf placed in 3% water agar in a 30-ml plastic cup, and a sporulating fungal mat was placed over the aphids to shower conidia onto them. After approximately 1 hr of incubation in light at 20oC, the sporulating mat was removed and the cup was sealed. Inoculated aphids were incubated at 20oC, with a 16 hr photoperiod and cups were checked on a daily basis. Sporulating cadavers were used in assays within eight hours after host death. Uninfected aphid cadavers. Uninfected aphid cadavers were produced by freezing healthy apterous adults and 4th instar nymphs at -20 oC for 10 min. Resulting cadavers were always used in assays within eight hours. Living aphids contaminated with conidia. Healthy apterous adults and 4th instar nymphs were transferred to a milkweed leaf placed in 3% water agar in a 30-ml plastic cup, after which a sporulating fungal mat was placed over the aphids. After 1-3 hr of incubation in light at 20oC, the sporulating mat was removed and the cup was sealed. The conidialshowered aphids were always used within five hours after conidial showering. For all treatments experimental aphids were placed in 29 ml plastic cups after production. Cups contained 3% water agar to avoid dehydration and for living experimental aphids cut milkweed leaves were provided as food. Cups containing experimental aphids were transported to the field in a cooler. Field methods Experimental aphids were carefully introduced into colonies of the milkweed aphid using a fine paintbrush. The first set of treatments (2005) had three types of experimental aphids (sporulating cadaver, cadaver of an aphid killed by freezing, living aphid) and the second set of treatments (2007) had two types (living conidia-contaminated aphid, living uncontaminated aphid). For each replicate, a set of treatments was introduced to the same colony on the same day. For each set of treatments, one type of experimental aphid was introduced to a colony at a time, with at least 30 min before a different type of experimental aphid was introduced. Timing was initiated as soon as an ant entered the aphid colony. The experimental aphid was carefully observed continuously by one person for five min while ant behaviour was recorded. Removal of aphids or aphid cadavers by ants was recorded as follows: (i) the experimental aphid was carried down the plant stem to the ground, (ii) the experimental aphid was flung from the side of a leaf, or (iii) the experimental aphid was deposited more than 1 cm away from the colony on the same food plant. Each experiment was conducted for five minutes or until the experimental aphid was removed. For studies with conidia-contaminated aphids, ant behaviour after each tending event was categorized as (i) the ant removed the experimental aphid, (ii) the ant picked up and groomed the experimental aphid, (iii) the ant groomed itself, or (iv) the ant did not take any action and moved on to tending other aphids in the colony. Data analysis Removal of experimental aphids was analyzed using a Cox proportional-hazard regression model, with treatment, aphid colony size and number of tending ants in the aphid colony used as dependent variables. The removal distributions were analyzed using Kaplan-Meier survival tests with the Breslow statistic (Allison, 1995). Data from studies exploring responses to sporulating cadavers versus conidia-contaminated living aphids were analyzed separately. Multiple regressions were used to analyze the effect of types of experimental aphid on the number of touches by ants, the number of times that ants picked up aphids, the number of tending events and tending time, with treatment and number of tending ants in the aphid colony used as dependent variables (Proc GLM; SAS Institute, 1999). The effect of types of experimental aphids introduced to the colony on the proportion of experimental aphids that ended on the ground was analyzed by logistic regression with treatment, aphid colony size and number of tending ants in the aphid colony as dependent variables (Proc GENMOD; SAS Institute, 1999). Logistic regression was used to analyze ant responses after tending (N=210), with a multinomial distribution and cumulative logit as a link function (four categories: self-grooming, aphid-grooming, removing experimental aphid and no reaction) with treatment as a dependent variable (Proc GENMOD; SAS Institute 1999). Allison, P. D. 1995. Survival Analysis using SAS. A practical Guide. SAS Institute Cary, NC: SAS Publ. Papierok, B. & Hajek, A. E. 1997 Entomophthorales. In Manual of Techniques in Insect Pathology (ed. L. A. Lacey), pp. 187-212. London: Academic Press. SAS Institute. 1999 SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Publ.