139KB - NZQA
advertisement

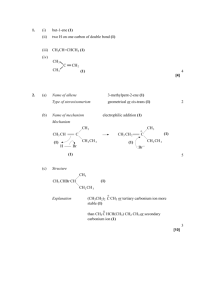
NCEA Level 2 Chemistry (90309) 2010 — page 1 of 4 Assessment Schedule – 2010 Chemistry: Describe the structural formulae and reactions of compounds containing selected organic functional groups (90309) Evidence Statement Q ONE (a) Evidence Achievement Structural formula IUPAC name TWO of CH3CH2CH2CH2COOH pentanoic acid CH3CH2COOCH2CH3 ethyl propanoate • THREE names or structures correct. 3-methylbut-1-yne Achievement With Merit Achievement with Excellence Three linked explanations of (1), (2), (3), (4). A full discussion of structural isomers, linked to molecules C and D, AND geometric isomers linked to the structure of molecule A. 2-chlorobutan-2-ol (b)(i) C and D (1) They have the same molecular formula C7H14, the same number of carbon and hydrogen atoms, but their atoms are arranged differently. (ii)(1) (2) A (cis-hex-2-ene) (trans-hex-2-ene) (3) (2) Cis-trans isomers occur in molecules that have carbon-to-carbon double bonds because rotation of the atoms about the axis of the carbon-to-carbon double bond is restricted. (3) They must also have two different groups attached to each of the carbons involved in the double bond. (4) Molecule A, hex-2-ene, has a double bond. It has two different groups attached to the carbons, i.e. one carbon of the double bond is attached to H and CH3 and the other carbon is attached to H and CH2CH2CH3. • Correct structural isomers identified. • Molecule that can exist as cistrans isomers identified and isomers drawn. NCEA Level 2 Chemistry (90309) 2010 — page 2 of 4 TWO (a)(i) CH3 – CH = CH2 Elimination THREE of • TWO products or two reaction types. (ii) BOTH of Two products identified and linked to reaction type. (Two pairs, reaction type and structure correct.) Esterification/elimination (iii) • Polymer drawn correctly. (b) (c) AND CH3–COOK or CH3–COO–K+ Acid-base Both reactions are addition reactions. This involves breaking the double bond between the carbon atoms in but-1-ene and forming a single bond in its place, as well as forming two new single bonds. With Cl2, the new single bonds formed are both C–Cl bonds. With HCl, the new single bonds are a C–Cl bond and a C–H bond. • Description of addition reaction, or recognises Markovnikov’s rule. With Cl2 But-1-ene reacts to form one product CH3–CH2–CH=CH2 + Cl2 CH3–CH2–CHCl–CH2Cl • One product for but-1-ene with either Cl2 or HCl. EITHER But-1-ene reacts with Cl2 to form CH3-CH2-CHClCH2Cl. Linked to an addition reaction. But-1-ene reacts with Cl2 to form CH3–CH2–CHCl– CH2Cl OR AND With HCl But-1-ene reacts to form two products. This is because but-1-ene is an asymmetric alkene (one carbon atom of the double bond has different groups attached to it). CH3–CH2–CHClCH3 is the major product because the H from HCl will bond to the carbon atom with the greatest number of H atoms. CH3–CH2–CH=CH2 + HCl CH3–CH2–CHClCH3 (major product) CH3–CH2–CH2–CH2Cl (minor product) But-1-ene reacts with HCl to form CH3–CH2– CHClCH3 (major product) and CH3–CH2–CH2CH2Cl (minor product). Linked to an addition reaction. But-1-ene reacts with HCl to form CH3–CH2– CHClCH3 (major product) and CH3–CH2–CH2– CH2Cl (minor product) Linked to an explanation of an addition reaction (breaking of double bond and drawing correct structures suffices here) and the formulae of products. NCEA Level 2 Chemistry (90309) 2010 — page 3 of 4 THREE (a)(i) (ii) (b)(i) (ii) Secondary. The carbon that the OH group is attached to, is attached to two other carbons. TWO of • One alcohol correctly classified and the reason described. Primary. The carbon that the OH group is attached to, is attached to only one other carbon. One of these groups circled: • Functional group correct. Bromine water rapidly decolourised from orange to colourless. • Observation described. (iii) • ONE correct product for hydrolysis reaction. TWO correct products for hydrolysis reaction. FOUR Add Na2CO3 to a sample of each organic substance. The substance which produces bubbles is the propanoic acid. The bubbles are CO2. 2CH3CH2COOH + Na2CO3 2CH3CH2COONa + CO2 + H2O Add MnO4-/H+ to the two remaining samples. One of the two samples will turn the solution from purple to colourless. This is hex-1-ene. The organic substance that does not change the colour of acidified potassium permanganate nor reacts with sodium carbonate is hexane. One molecule identified with correct reagent and observation. Two molecules identified with correct reagent and observation. (Allow a statement that hexane does not react with either reagent as indentifying a molecule). OR One molecule identified with correct reagent, observation and equation. THREE correct products for hydrolysis reaction. Valid method that identifies each sample. This includes reagent used, observations and equations. NCEA Level 2 Chemistry (90309) 2010 — page 4 of 4 Judgement Statement Achievement Achievement with Merit Achievement with Excellence 3A OR 1M/E+1A 3M 3E