February 2014 - The Chicago Pathology Society
advertisement

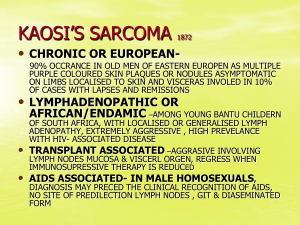
ILLINOIS REGISTRY OF ANATOMIC PATHOLOGY CASE HISTORIES AND DIAGNOSES FEBRUARY 24, 2014 Case #1 PRESENTER: Chelsea Curry, D.O., M.B.A. ATTENDING: William G. Watkin, M.D. CASE HISTORY: The patient is a 57 year male who presented with several weeks of frequent and painful urination. The symptoms did not resolve with antibiotics. His past medical and surgical history are unremarkable. DIAGNOSIS: Encrusted cystitis IMPORTANT DIFFERENTIAL DIAGNOSES: Schistosomiasis, cyclophosphamide-induced cystitis, tuberculous cystitis, foreign body cystitis KEY DIAGNOSTIC FEATURES: Rare condition most commonly caused by Corynebacterium urealyticum, a Gram positive bacillus with urease activity that hydrolyzes urea causing the release of ammonia which alkalinizes the urine o Can also be caused by other urea-splitting bacteria This process facilitates bacterial adherence, tissue inflammation, and the precipitation of calcium phosphate and ammonium, magnesium, and phosphate salts (struvite) o The precipitation of these crystals leads to the bladder wall encrustations Usually occurs in patients with predisposing risk factors such as: o Preexisting lesion of the bladder mucosa secondary to a prior urologic procedure or prolonged bladder catheterization o Long-term hospitalization with broad-spectrum antibiotic therapy o Immunosuppressive therapy following renal transplantation or for a systemic inflammatory condition Histology: o Ulcerated, necrotic mucosa with calcifications o Inflammatory cells including lymphocytes, neutrophils, and/or eosinophils Urine culture: o Corynebacterium species can be missed on routine cultures o Growth requires enriched media and prolonged incubation for more than 48 hours o Molecular testing can aid in organism identification Treatment: o Antimicrobial therapy (glycopeptides - typically vancomycin or teicoplanin) o Urine acidification (oral or topical acidic solutions) o Endoscopic removal of encrustations (often needed multiple times) REFERENCES: Lieten S, Schelfaut D, Wissing KM, Geers C, Tielemans C. Alkaline-encrusted pyelitis and cystitis: an easily missed and life-threatening urinary infection. BMJ Case Rep 2011; 2011: bcr1220103613. Published online March 29, 2011. Soriano F, Tauch A. Microbiological and clinical features of Corynebacterium urealyticum: urinary tract stones and genomics as the Rosetta Stone. Clin Microbiol Infect 2008; 14(7): 632-643. Johnson MH, Strope SA. Encrusted cystitis. Urology 2012; 79(3): e31-e32. Guimaraes LC, Soares SC, Albersmeier A, et al. Complete genome sequence of Corynebacterium urealyticum strain DSM 7111, isolated from a 9-year-old patient with alkalineencrusted cystitis. Genome Announc 2013; 1(3): e00264-13. Published online May 23, 2013. Perciaccante A, Pompeo ME, Fabi F, Venditti M. Successful treatment of Corynebacterium urealyticum encrusted cystitis: a case report and literature review. Le Infezioni in Medicina 2007; 1: 56-58. Pagnoux C, Berezne A, Damade R, et al. Encrusting cystitis due to Corynebacterium urealyticum in a patient with ANCA-associated vasculitis: case report and review of the literature. Semin Arthritis Rheu 2011; 41(2): 297-300. Meria P, Desgrippes A, Arfi C, Le Duc, A. Encrusted cystitis and pyelitis. J Urol 1998; 160: 3-9. Galan-Sanchez F, Aznar-Marin P, Marin-Casanova P, Garcia-Martos P, Rodriguez-Iglesias M. Urethritis due to Corynebacterium glucuronolyticum. J Infect Chemother 2011; 17: 720-721. Devriese J, Riegel P, Hommez J, Vaneechoutte M, De Baere T, Haesebrouck F. Identification of Corynebacterium glucuronolyticum strains from the urogenital tract of humans and pigs. J Clin Microbiol 2000; 38(12); 4657-4659. Prete DD, Polverino B, Ceol M, et al. Encrusted cystitis by Corynebacterium urealyticum: a case report with novel insights into bladder lesions. Nephrol Dial Transplant 2008; 23: 2685-2687. Tanaka T, Yamashita S, Mitsuzuka K, et al. Encrusted cystitis causing postrenal failure. J Infect Chemother 2013; 19: 1193-1195. Case #2 PRESENTER: Garrison Pease, M.D. ATTENDING: Barbara Golden, M.D. Clinical: A 27 year old Caucasian woman presents with a one-month history of flank pain, N/V, fatigue and constipation unresolved by medication including antibiotics. Imaging studies show left renal hilar adenopathy and a 2.6 cm mass in the upper pole and medulla of the kidney. A nephrectomy is performed. Histopathology: Multinodular, sheeting growth pattern. Vesicular nuclei and large eccentric nucleoli, with densely eosinophilic cytoplasmis inclusions pushing nucleus to the side. Special studies: CK8/CK18, OSCAR, and EMA positive. Differential diagnosis: RCC (sarcomatoid, rhabdoid, NOS), epithelioid sarcoma, epithelioid angiosarcoma, renal medullary carcinoma, rhabdoid tumor, epithelioid hemangioendothelioma. Further special studies: ERG, CD31, and CD34 positive. INI-1 expression lost. PAX2 and PAX8 negative. CD99 and desmin negative. FISH gene analysis performed. With overlapping histopathology in the tumors on the differential diagnosis, special studies help narrow the possibilities: Patient’s demographics (race, lack of Sickle trait/disease), PAX8 negativity, and CD34 positivity decrease likelihood of renal medullary carcinoma. INI-1 lost expression and PAX8 negativity help rule out RCCs. While vascular marker positivity (CD31 and ERG) suggests a vascular etiology, studies show that another tumor in the differential (epithelioid sarcoma) can express vascular/endothelial markers. INI-1 expression would be maintained in epithelioid angiosarcoma and hemangioendothelioma. With INI-1 expression lost in both remaining differential diagnoses, CD34 positivity, patient’s age, and gene preservation by FISH help rule out rhaboid tumor. This leaves epithelioid sarcoma standing highest on the differential. Generally it shows variable cytokeratin positivity, variable vascular endothelial/vascular positivity, lost INI-1 expression, and variable gene mutations/deletions by FISH. Epithelioid sarcoma, distal type: Mean age 26, more likely male. Distal (classic type) tends to occur at distal extremities. Can be confused with a granulomatous pattern, with epithelioid and spindled cells. Epithelioid sarcoma, proximal type: Slightly older age, tends to occur at proximal locations such as vulva, pubis, penis. Larger epithelioid and rhabdoid cells in sheeting pattern. ***Diagnosis: Epithelioid sarcoma, proximal type.*** Brief introduction to INI-1: Tumor suppressor gene on ch22 Heterozygous deletions, or total ablation of the gene, in mice allow tumors with rhabdoid morphology to develop. Lost expression Retained expression Renal medullary carcinoma Epithelioid angiosarcoma Malignant rhabdoid tumor Epithelioid hemangioendothelioma Epithelioid sarcoma Metastatic melanoma Synovial sarcoma (incomplete loss) Epithelioid mesothelioma For epithelioid sarcoma, INI-1 gene loss (FISH) in ~50% of cases that show lost INI-1 expression by IHC; Rhabdoid tumor has 98% rate of gene loss by FISH. Key points in narrowing the differential toward epithelioid sarcoma: RCCs, especially renal medullary carcinoma, can be ruled out by a combination of IHC, patient demographics, and comorbidities. Vascular endothelial cell tumors cannot reliably be ruled out by IHC. INI-1 expression vs. loss helps to differentiate tumors with rhabdoid morphology. o Presence of absence of INI-1 gene helps further. Proximal type epithelioid sarcoma is a newer form of epithelioid sarcoma, occurring at a slightly older age, and at more proximal anatomic locations. References: Eaton KW, Tooke LS, Wainwright LM, Judkins AR, Biegel JA. Spectrum of SMARCB1/INI1 Mutations in Familial and Sporadic Rhabdoid Tumors. Pediatr Blood Cancer. 2011 Jan;56(1):715. PMID: 21108436 Hollmann TJ, Hornick JL. INI1-Deficient Tumors: Diagnostic Features and Molecular Genetics. Am J Surg Pathol. 2011 Oct;35(10):e47-63. PMID: 21934399 Guillou L, Wadden C, Coindre JM, Krausz T, Fletcher CD. “Proximal-type” Epithelioid Sarcoma, a Distinctive Aggressive Neoplasm Showing Rhabdoid Features: Clinicopathologic, Immunohistochemical, and Ultrastructural Study of a Series. Am J Surg Pathol. 1997 Feb;21(2):130-46. PMID: 9042279. Miettinen M, Fanburg-Smith JC, Virolainen M, Shmookler BM, Fetsch JF. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999 Aug;30(8):934-42. PMID: 10452506 Chbani L, Guillou L, Terrier P, Decouvelaere AV, Grégoire F, Terrier-Lacombe MJ, Ranchère D, Robin YM, Collin F, Fréneaux P, Coindre JM. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French sarcoma group. Am J Clin Pathol. 2009 Feb;131(2):222-7. PMID: 19141382 Cheng JX, Tretiakova M, Gong C, Mandal S, Krausz T, Taxy JB. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008 Jun;21(6):647-52. PMID: 18327209 Hasegawa T, Matsuno Y, Shimoda T, Umeda T, Yokoyama R, Hirohashi S. Proximal-type epithelioid sarcoma: a clinicopathologic study of 20 cases. Mod Pathol. 2001 Jul;14(7):655-63. PMID: 11454997 Gasparini P, Facchinetti F, Boeri M, Lorenzetto E, Livio A, Gronchi A, Ferrari A, Massimino M, Spreafico F, Giangaspero F, Forni M, Maestro R, Alaggio R, Pilotti S, Collini P, Modena P, Sozzi G. Prognostic determinants in epithelioid sarcoma. Eur J Cancer. 2011 Jan;47(2):287-95. PMID: 20932739 Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003 Sep;127(9):1161-8. PMID: 12946229 Stockman DL, Hornick JL, Deavers MT, Lev DC, Lazar AJ, Wang WL. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2013 Sep 27. doi: 10.1038/modpathol.2013.161. [Epub ahead of print]. PMID: 24072183 Miettinen M, Wang Z, Sarlomo-Rikala M, Abdullaev Z, Pack SD, Fetsch JF. ERG expression in epithelioid sarcoma: a diagnostic pitfall. Am J Surg Pathol. 2013 Oct;37(10):1580-5. PMID: 23774169 Case #3 PRESENTER: Michael Eckhardt, M.D. ATTENDING: Thomas Cibull, M.D. HISTORY: 33 year old male, status post renal transplant 1 year ago, who presents with a 1 month history of multiple asymptomatic discrete vascular to keratotic and scaly papules on the extremities. Clinically the differential was bacillary angiomatosis versus Kaposi’s sarcoma and biopsies of the left forearm, left medial ankle and right anterior shin were taken. GROSS: Cylindrical piece of skin with discrete vascular papules. HISTOLOGY: Microscopically the lesion shows pseudoepitheliomatous hyperplasia with suppurative granulomatous inflammation. Round and elongated structures can be seen within the tissue. DIFFERENTIAL DIAGNOSIS: 1) Fungal infection 2) Atypical mycobacterium 3) Foreign body granuloma STAINS: PAS-D and GMS stains showed spores and septate and hyaline hyphae, Fontana Masson stain was negative for pigmentation of the hyphae, AFB stain was negative for mycobacterium. CULTURE: Paecilomyces lilacinus DIAGNOSIS: Paecilomyces lilacinus KEY POINTS: Paecilomyces is a filamentous fungus that rarely causes infection in humans Immunocompotent patients are more likely to develop ocular infections due to its tropism for ocular structures Immunocompromised patients are more likely to develop cutaneous infections Paecilomyces is resistant to conventional antifungals, so culture is critical for diagnosis and proper treatment REFERENCES: Aguilar, C. et al.: (1998). Antifungal susceptibilities of paecilomyces species. Antoimicrobial Agents and Chemotherapy , 42 (7), 1601-1604. Blackwell, V. et al.: (2000). Cutaneous hyalohyphomycosis caused by paecilomyces lilacinus in a renal transplant patient. British Journal of Dermatology , 143, 873-875. Harris, L. F., et al.: (1979). Paecilomyces cellulitis in a renal transplant patient: successful treatment with intravenous miconazole. Southern Mecical Journal , 72 (7), 897-898. Itin, P. e. (1998). Cutaneous manifestations of paecilomyces lilacinus infection induced by a contaminated skin lotion in patients who are severely immunosuppressed. Jounal of the American Academy of dermatology , 39, 401-409. Pastor, F. J. (2006). Clinical manifestations, treatment and outcome of paecilomyces lilacinus infections. Clinical Microbiology and Infection , 12 (10), 948-960. Schoonevald, T. V. (2008). Paecilomyces lilacinus infection in a liver transplant patient: case report and review of the literature. Transplant Infectious Disease , 10, 117-122. Case #4 PRESENTER: William Gibson, M.D. ATTENDING: Michael Kaufman, M.D. CASE HISTORY: A 42 year old Romanian woman presents to her annual physical with dull, intermittent abdominal pain for three months. Imaging reveals a 6.7 cm complex cystic mass in the right lobe of the liver. A cyst unroofing is performed. DIAGNOSIS: Cystic Echinococcosis CLINICAL DIFFERENTIAL: Biliary cystadenoma/carcinoma, biloma, cystic abscess, metastasis EPIDEMIOLOGY: 50 million affected (Africa, Europe, Asia, Mid-East, and Central and S. America) WHO neglected zoonotic disease, grossly underreported Rural, grazing areas where dogs ingest organs from infected animals PATHOPHYSIOLOGY: Eggs/larvae in intermediate host (sheep) Adults in definitive host (dogs) Eggs hatch in the duodenum, migrate through intestinal wall into mesenteric venules Become lodged in tissues - liver (70%), lung, bone, orbit, heart, brain DIAGNOSTIC FEATURES: History of travel to agrarian areas Serum antibody screening Liver cyst on CT/MRI/ultrasound Laminated layer on frozen section TREATMENT: Surgical: radical (pericystectomy) vs. conservative (unroofing) Percutaneous (PAIR) – aspiration and injection of scolicidal agent (NaCl/EtOH) Benzamidazole therapy (albendazole) PO x 4 weeks Give benzamidazole before surgery to prevent possible anaphylaxis Novel scolicidal agents: sambucus ebulus, garlic, squash seeds, hazelnut, berberis vulgaris VACCINATION: Sheep: Eg95, rEg14-3-3 Dog: EgM4-EgM9-EgM123, EgA31-EgTrp REFERENCES: Kim, AR, Park SJ, Gu, MJ, Choi, JH, Kim, HJ. Fine Needle Aspiration Cytology of Hepatic Hydatid Cyst: A Case Study. The Korean Journal of Pathology. 2013; 47: 395-398. Gruttadauria, S. Hepatic Echinococcis: Clinical and therapeutic aspects. World Journal of Gastroenterology. 2012; 18(13): 1448-1458. Rahimhesboei, SH, Ebrahimzadeh, MA, Pourhajibagher, M. In vitro effect of Sambucus ebulus on scolices of Hydatid cysts. European Review for Medical and Pharmacological Sciences. 2013; 17: 1760-1765. Jairajpuri, ZS, Jetley, S, Hassan, JM, Hussain, M. Hydatid disease in childhood: revisited report of an interesting case. J Parasit Dis. Dec 2012; 36(2): 265-268. Zheng, H, Zhang, W, Zhang, L, et al. The genome of the hydatid tapeworm echinococcus granulosus. Nature genetics. Oct 2013; 45(10): 1168-77. Salinas, JL, Herman, VG, Astuvika, J, et al. Long term albendazole effectiveness for hepatic cystic echinococcosis. Am J Trop Med Hyg. 2011; 85(6): 1075-1079. Zhang, W, McManus, DP et al. Immunology and Immunodiagnosis of Cystic Echinococcosis: An Update. Clinical and Developmental Immunology. 2012; 1-10. Zhang, W, McManus, DP et al. "Vaccination of Dogs against Echinococcus granulosus, the Cause of Cystic Hydatid Disease in Humans." The Journal of Infectious Diseases 2006; 194:966– 74 Moghadam, ZK, Ghaffarifar, F, Khalilpour, A, et al. IgG4 Detection of Echinococcus granulosus Paramyosin is a useful Diagnostic Test for Human Hydatidosis. Clinical and Vaccine Immunology. Apr 2013; 20(4): 501-505. Eckert, J, Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clinical Microbiology Reviews. Jan 2004; 17(1): 107-135. ZJ, L, Wang YN, Wang Q, Zhao W. Echinococcus granulosus 14-3-3 protein: a potential vaccine candidate against challenge with Echinococcus granulosus in mice. Biomed Envrion Sci. Jun 2012; 25(3): 352-8. Rouhani S, Salehi N, Kamalinejad M, Zayeri F. Efficacy of Berberis vulgaris Aqueous Extract on Viability of Echinococcus granulosus protoscolices. J Invest Surg. Aug 2103. Eskandarian AA. Scolicidal effects of squash seeds, hazel nut and garlic extracts on hydatid cyst protoscolices. J Res Med Sci. Nov 2012; 17(11): 1011-4. Beaver, PC, Jung, RC, Cupp, EW. Clinical Parasitology 9th edition. 1984. Felici et al. “Percutaneous drainage of echinococcal cysts (PAIR—puncture, aspiration, injection, reaspiration): results of a worldwide survey for assessment of its safety and efficacy.” Gut 2000;47:156-157. Otero-Abad, Belen and Paul R. Torgerson. "A Systematic Review of the Epidemiology of Echinococcosis in Domestic and Wild Animals." PLOS Neglected Tropical Diseases. Vol 7, Issue 6, June 2013. Petavy, A, Bosquet, G et al. "An Oral Recombinant Vaccine in Dogs against Echinococcus granulosus, the Causative Agent of Human Hydatid Disease: A Pilot Study." PLoS Neglected Tropical Diseases. 2008 January; 2(1): e125. Aleksi -Shihabi, A and EP Vidolin. "Cystic Echinococcosis of the Heart and Brain: A Case Report." Acta Me. Okayama, 2008. Vol. 62, No. 5, pp. 341-344. Medscape.com: Echinococcus granulosus, accessed October 25th, 2013. http://www.cdc.gov/parasites/echinococcosis/gen_info/ce-faqs.html http://www.vascularinterventions.net/procedures/non-vascular-procedures/rfa-for-lung-liver-andbone-lesions/ PAIR: Puncture, Aspiration, Injection, Re-Aspiration: An option for the treatment of Cystic Echinococcosis. World Health Organization. http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_APH_2001.6.pdf Akinci OF et al. "In vitro efficacy of different chemical substances on hydatid cyst components." Turkish Journal of Medical Science. 2011;41(1):17-23. Case #5 PRESENTER: Zachary Michalicek, D.O. ATTENDING: John Lee M.D., Ph.D. CASE HISTORY: A 36 year old G6P3 female presented to the emergency department for headaches one month after cesarean section with spinal anesthesia. Magnetic resonance imaging demonstrated a rapidly growing, T1 isointense/decreased T2 signal sellar mass with extension into the suprasellar cistern. DIAGNOSIS: Adult teratoid/rhabdoid tumor (AT/RT) IMPORTANT DIFFERENTIAL DIAGNOSIS: PNET/extra-osseous Ewing sarcoma, rhabdomyosarcoma, lymphoma, KEY DIAGNOSTIC FEATURES: Cellular neoplasm composed of a variable mix of rhabdoid cells, epithelial cells, primitive neuroectodermal cells and mesenchymal elements Loss of INI-1 positivity is a sensitive and specific marker for this entity Most commonly found in the posterior fossa of children May be found in the supratentorial, infratentorial or spinal cord regions in adults SPECIAL STUDIES: A loss of staining for INI-1 is a sensitive and specific marker A majority of cases show deletion or mutation of the INI1/hSNF5 tumor suppressor gene on chromosome 22q11 This tumor is positive for vimentin and CD99 Staining for desmin is negative Stains with variable positivity include SMA, EMA, kertin, synaptophysin and NSE REFERENCES: Biegel, Jaclyn A., Benjamin Fogelgren, Jun-Ying Zhou, et al. "Mutations in the INI1 Rhabdoid Tumor Suppressor Gene in Medulloblastomas and Primitive Neuroectodermal Tumors of the Central Nervous System." Clinical Cancer Research 6 (2000): 2759-763. PubMed. Web. Las Heras, Facundo, and Kenneth P.H. Pritzker. "Adult Variant of Atypical Teratoid/rhabdoid Tumor: Immunohistochemical and Ultrastructural Confirmation of a Rare Tumor in the Sella Tursica." Pathology - Research and Practice 206 (2010): 788-91. PubMed. Web. Makuria, Addisalem T., Elisabeth J. Rushing, Kevin M. McGrail, et al. "Atypical Teratoid Rhabdoid Tumor (AT/RT) in Adults: Review of Four Cases." Journal of Neuro-Oncology 88 (2008): 32130.PubMed. Web. Moretti, Costanzo, Domenico Lupoi, Francesca Spasaro, et al. "Sella Turcica Atypical Teratoid/Rhabdoid Tumor Complicated with Lung Metastasis in an Adult Female."Clinical Medicine Insights 6 (2013): 177-82. PubMed. Web. Schneiderhan, T. M., K. Beseoglu, M. Bergmann, et al. "Sellar Atypical Teratoid/rhabdoid Tumors in Adults." Neuropathology and Applied Neurobiology 37 (2011): 326-29.PubMed. Web. Shonka, Nicole A., Terri S. Armstrong, Sujit S. Prabhu, et al. "Atypical Teratoid/rhabdoid Tumors in Adults: A Case Report and Treatment-focused Review." Journal of Clinical Medicine Research 3.2 (2011): 85-92. Web. "SMARCB1." Genetics Home Reference. National Institute of Health, May 2013. Web. <http://ghr.nlm.nih.gov/gene/SMARCB1>. Case #6 PRESENTER: Sharda Thakral, M.D. ATTENDING: Wayne Wirtz, M.D. CLINICAL HISTORY: A 48 year old male presented with a one year history of intermittent leftsided groin pain and an increase in size of his left testicle. Imaging by U/S showed no intratesticular mass. Serum LDH, AFP, and HCG were all within the normal range. Surgical exploration led to the resection of a 10 cm left scrotal wall mass. IMMUNOHISTOCHEMISTRY: Positive stains: CD 34, ER, SMA (spindle cells weakly positive), caldesmon (spindle cells weakly positive) Negative Stains: S-100, desmin FINAL DIAGNOSIS: Angiomyofibroblastoma-like tumor of the male genital tract DIFFERENTIAL DIAGNOSIS: Diagnosis Clinical Histopathology IHC Spindle cell lipoma Well circumscribed Neck, shoulder, back Short bundles of eosinophilic, ropy collagen Inconspicuous vessels Fat present CD 34 + Desmin – ER/PR – Solitary fibrous tumor Well circumscribed Variety of locations including vulva, perineum Hypocellular/ hypercellular areas Hemangiopericytoma-like vessels CD 34 + Desmin – ER/PR – Mammary-type myofibroblastoma Well circumscribed Can be extra-mammary including male inguinal region Inconspicuous vessels CD 34 + Desmin + ER/PR – Angiomyofibroblastoma Well circumscribed Thin, fibrous capsule Vulva, scrotum Peri-vascular accentuation of tumor cells Binucleate, multinucleate CD 34 + Desmin + ER/PR + Aggressive angiomyxoma Infiltrative margins Vulvo-vaginal, male inguinal region 30% local recurrence Smooth muscle cells “spinning off” of blood vessels CD 34 + Desmin + ER/PR + DISCUSSION: Angiomyofibroblastoma-like tumor of the male genital tract/ Cellular Angiofibroma Clinical: Rare, benign tumor Excision is curative Sites of involvement: Superficial soft tissues of vulva or inguinoscrotal region, perineum, retroperitoneum, chest Gross: Well-circumscribed mass Cut surface yellow to tan-brown, soft to rubbery Generally <3 cm in diameter in female and <10 cm in male Histological features: Plump, spindled cells: Minimal eosinophilic cytoplasm Little cytological atypia Background: Delicate, wispy collagen Small to medium vessels, may have prominent hyaline walls Mast cells often present Fat may be present REFERENCES: Emtage JB, Parker J, Marcet JE, et al. A large cellular angiofibroma of the male pelvis presenting with obstructive voiding: A case report and review of the literature. Can Urol Assoc J 2013;7:E373-5. Flucke U, van Krieken JHJM, Mentzel T. Cellular angiofibroma: analysis of 25 cases emphasizing its relationship to spindle cell lipoma and mammary-type myofibroblastoma. Modern Pathol 2011;24:82-9. Ito M, Yamaoka H, Sano K, et al. Angiomyofibroblastoma of the male inguinal region. Arch Pathol Lab Med 2000;124:1679-81. Kumarapeli AR, Paczos T, Azabdaftari G. Morphological and immunohistochemical features of angiomyofibroblastoma: a case report with review of the literature. N Am J Med Sci 2011;4:100103. Laskin WB, Fetsch JF, Mostofi FK. Angiomyofibroblastomalike tumor of the male genital tract; analysis of 11 cases with comparison to female angiomyofibroblastoma and spindle cell lipoma. Am J Surg Pathol 1998;22:6-16. Lee SH, Yang JW, Do JM, et al. Angiomyofibroblastoma-like tumor of the scrotum. Korean J Urol 2010;51:365-7. McMenamin ME, Fletcher CDM. Mammary-type myofibroblastoma of soft tissue. A tumor closely related to spindle cell lipoma. Am J Surg Pathol 2001;25:1022-9. Nucci MR, Granter SR, Fletcher CDM. Cellular angiofibroma: a benign neoplasm distinct from angiomyofibroblastoma and spindle cell lipoma. Am J Surg Pathol 1997;21:636-44.